If you conduct studies, serve as a peer reviewer, and/or read publications that use connected sensor technologies, the EVIDENCE checklist is for you!
EValuatIng connecteD sENsor teChnologiEs
(EVIDENCE) Checklist
Reporting in peer reviewed literature evaluating digital measurement products is highly variable creating low confidence in results and researchers unnecessarily repeating work. To speed the development and deployment of digital measurement products worthy of our trust, the quality of reporting in published literature must improve.
The EVIDENCE checklist promotes high-quality reporting in studies where the primary objective is an evaluation of a digital measurement product or its constituent parts. The EVIDENCE checklist and manuscripts were developed by participants in a DiMe project and were published on May 18, 2021.
This project developed the Evidence Checklist to raise the bar for quality reporting of digital measurement product evaluation in the published literature. It is constructed similarly to existing publication checklists such as PRISMA for systematic reviews and meta-analyses, CONSORT for randomized clinical trials.
The 25-item checklist covers universal requirements for best research practices plus unique considerations for reporting on connected sensor technologies and software. Check out the manuscript published in Digital Biomarkers for further details and examples.
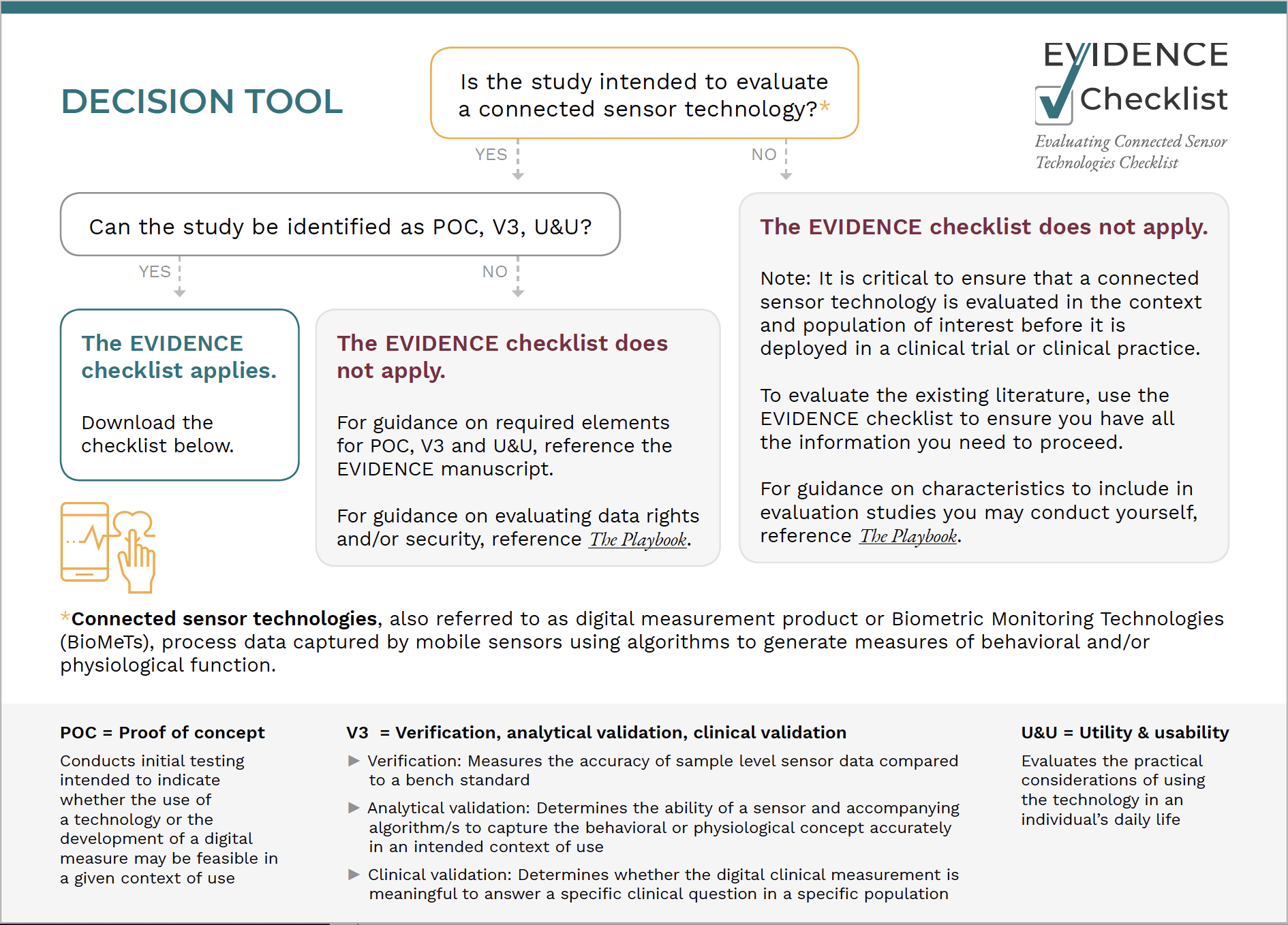
Does EVIDENCE apply to your study?
Use our decision algorithm to decide!
Are you a journal editor interested in endorsing EVIDENCE? Contact us.
Have you published a study using EVIDENCE? Share it with us.
Did you miss the launch event? Watch it now
Meet the experts who developed the EVIDENCE checklist
A group of 12 experts with different disciplinary backgrounds collaborated to develop the EVIDENCE Checklist. This sprint team represented experts from a variety of different work settings and multiple regulatory and geographic regions. Meet the team:
Christine Manta, Nikhil Mahadevana, Jessie Bakker, Simal Ozen Irmak, Elena Izmailova, Siyeon Natalie Park, Jiat-Ling Poon, Santosh Shevade, Sarah Valentine, Benjamin Vandendriessche, Courtney Webster, and Jennifer C. Goldsack