Chronic respiratory diseases such as COPD and asthma affect more than 300 million people globally and are among the top causes of death worldwide. As clinicians and researchers seek new approaches to assessing patient respiratory health remotely, Strados Labs has focused on developing new ways to objectively monitor symptoms that these patients experience. Their innovative RESP®️ Biosensor is an FDA 510(k) cleared wearable device that continuously captures lung sound symptoms, including coughs, wheezes, and crackles, in a patient’s daily life. This advanced monitoring can lead to novel insights into treatment response and earlier detection of exacerbations, ultimately helping to prevent unnecessary hospitalizations.
The challenge
In 2023, as Strados Labs engaged with sponsor organizations’ clinical trial teams, they faced a recurring challenge: effectively communicating the regulatory clearances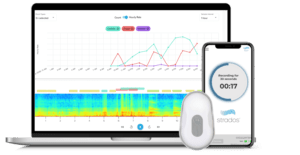 and validation work behind their RESP Biosensor. With an FDA 510(k) clearance to record lung sounds, including coughs, the RESP Biosensor had undergone extensive validation to meet clinical trial performance standards. However, the need to clearly differentiate between the device’s FDA clearance and the company’s clinical validation of its machine-learning algorithms became apparent.
and validation work behind their RESP Biosensor. With an FDA 510(k) clearance to record lung sounds, including coughs, the RESP Biosensor had undergone extensive validation to meet clinical trial performance standards. However, the need to clearly differentiate between the device’s FDA clearance and the company’s clinical validation of its machine-learning algorithms became apparent.
The solution
Strados Labs turned to DiMe’s The Playbook: Digital Clinical Measures to help bridge this communication gap. As they engaged with more teams from sponsor organizations, they noticed that many of the same questions were being asked about regulatory clearances, the validity of their measures, and the differentiation between data validation and regulatory clearance. The Playbook emerged as the perfect tool to clarify these critical points and refine Strados Labs’ communication of regulatory requirements.
“The Playbook has been an invaluable resource for us in conversations with clinical trial teams at life sciences organizations,” said Nick Delmonico, CEO of Strados Labs. Using The Playbook, Strados Labs could clearly demonstrate the regulatory clearance of their device and the validation work that supported its performance. This approach ensured that sponsor organizations fully understood the FDA framework for evaluating wearable sensors and digital measures, making them more confident in adopting the RESP technology in their clinical trials.
Incorporating The Playbook into its communications strategy was vital for Strados Labs to effectively frame conversations around its technology. This allowed Strados Labs to build trust and confidence with sponsor organizations while ensuring they fully understood the FDA framework for evaluating wearable sensors and digital measures.
Looking ahead
Strados Labs’ success in leveraging The Playbook highlights the critical role that structured frameworks play in navigating the complex regulatory landscape of digital health. The framework not only empowered their team to communicate more effectively but also equipped their customers with the knowledge needed to integrate cutting-edge technology confidently into their clinical trials.
As Strados Labs continues to advance respiratory health through innovative solutions, The Playbook will remain a crucial resource, guiding them in their mission to enhance patient outcomes and improve the quality of life for those living with chronic respiratory diseases.
About Strados Labs
Strados Labs is a medical technology company focused on improving the lives of patients with respiratory disease through innovative technology. Enabling real-world monitoring of lung health, Strados Labs’ RESP Biosensor is a wearable device that allows clinicians to remotely detect changes in patient symptoms, such as cough and wheeze that can indicate worsening health. The technology is used to assist clinical trials in the development of new therapies and to support healthcare teams in better managing patients with COPD, Asthma, and other cardiopulmonary diseases. The RESP Biosensor is FDA-cleared, CE marked and HIPAA compliant, backed by multiple awards and clinical publications. Strados Labs is privately owned and based in Philadelphia, PA.
For more information, visit Stradoslabs.com or follow us on LinkedIn and Twitter.