Federal Comment Toolkit
Optimizing Digital Medicine Regulations
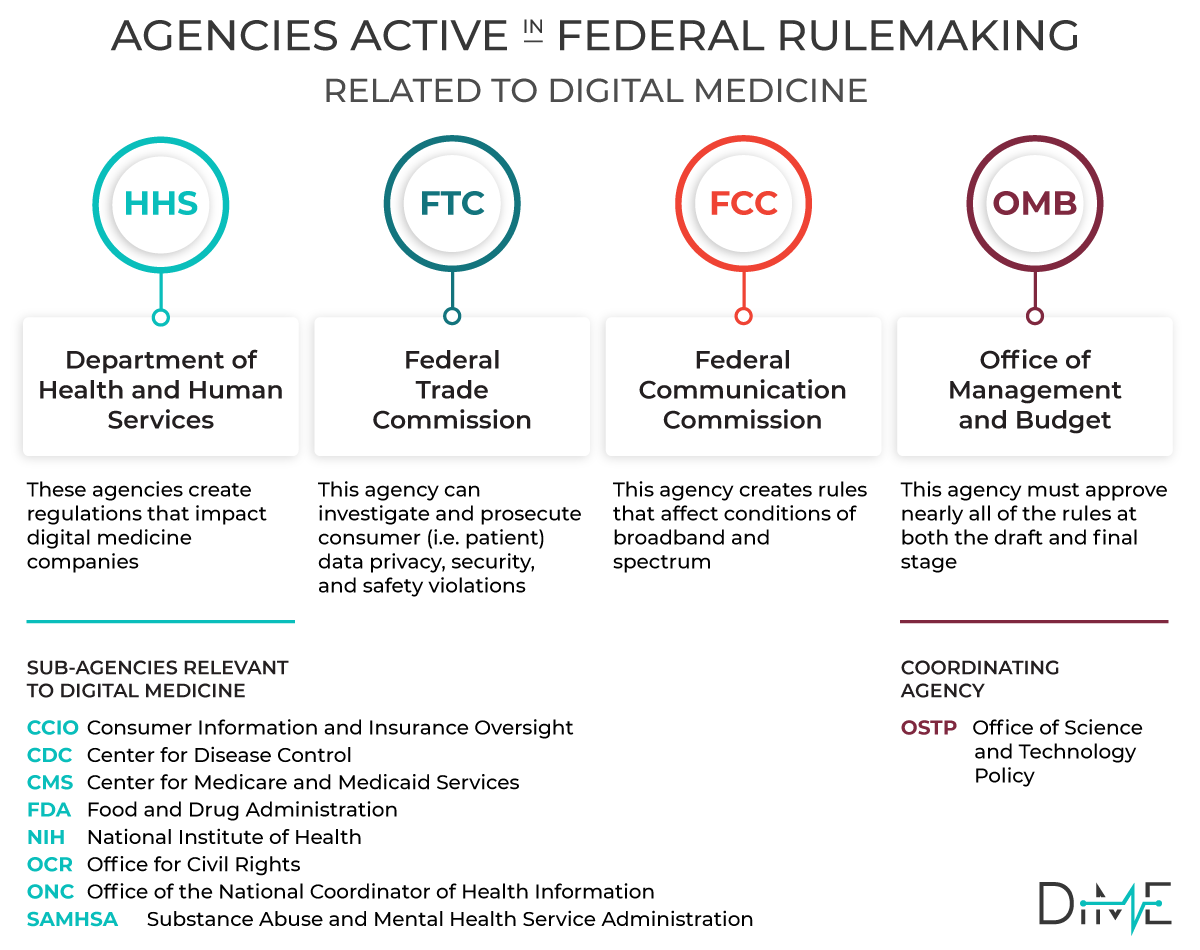
When a Federal Agency issues regulations —also known as ‘rules’ — they solicit public input to the process. Whether an Agency is responding to a new law or simply seeking to clarify a position or process, they are legally bound to seek public input.
- The Agency may open a public docket to seek early input.
- Any new rule or changes to an existing rule must be posted in draft form for public comment.
Federal agencies are required to consider all public comments when preparing final rules, so the public comment is a powerful opportunity to affect regulatory procedure. In addition, when Congress considers new legislation, they typically put out a request for information (RFI).
Your insight matters
DiMe has established a process to collate regulations that impact our field of digital medicine. Our goal is to make it easy for our community of experts to share their insights and provide broad expert input to the next generation of digital regulation in healthcare.
-
Please view our lists, which are searchable by keyword, agency, sub-agency, and RIN number.
-
Looking to collaborate on a public comment? Head to the DiMe Slack channel #federalcomment to find collaborators.
Rulemaking stages

What is coming up for comment?
Need help making your comment?
Check out previous comments or use one of our templates when you are ready to write your own comment.



