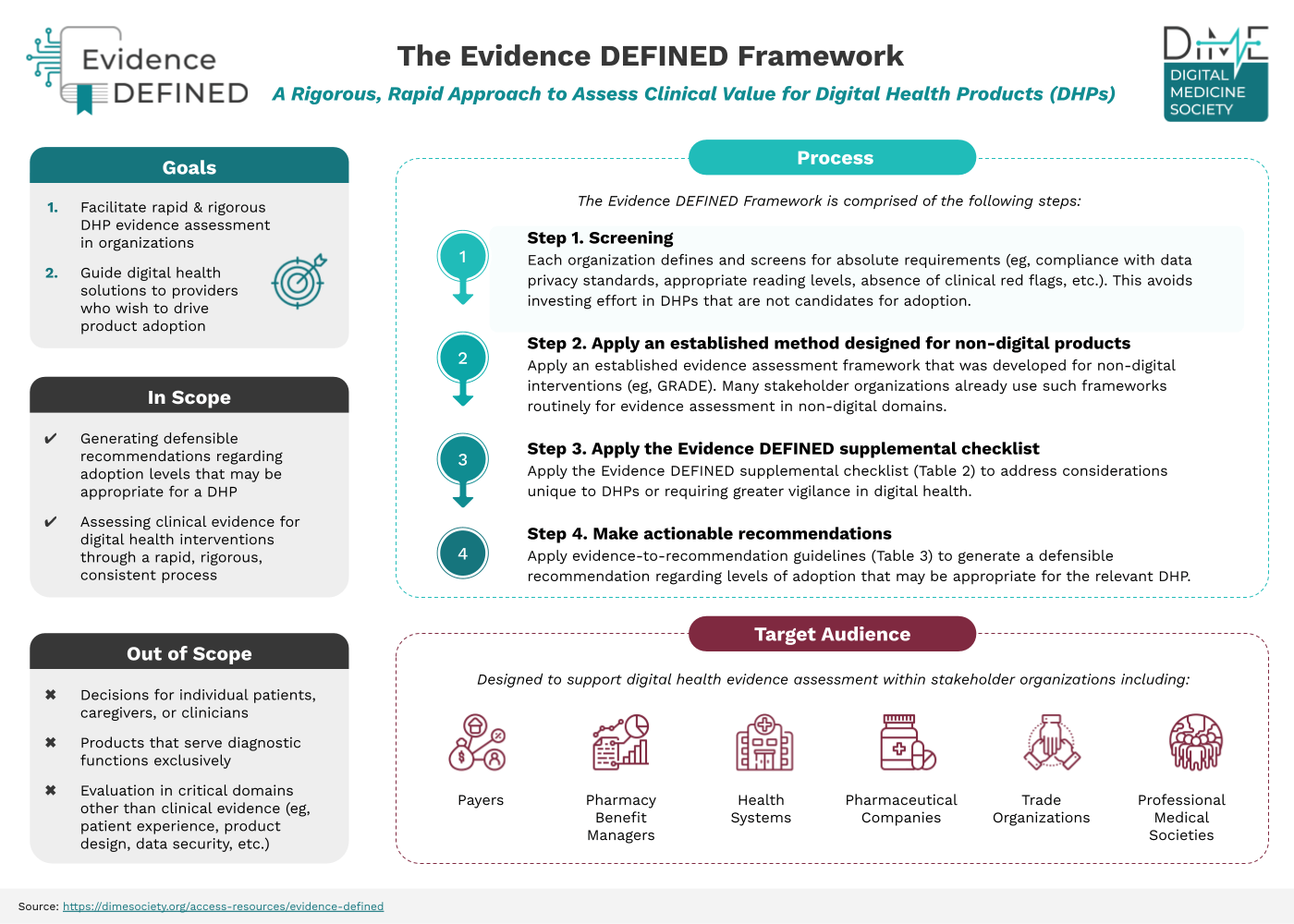
Evidence in Digital Health for EFfectiveness of INterventions with Evaluative Depth (Evidence DEFINED)
The new standard of excellence framework for clinical assessment of digital health products (DHPs).
Without the proper evidence for DHPs, stakeholders across the healthcare ecosystem, including payers, will encounter obstacles that can result in companies being unable to bring their products to market. Evidence is needed to determine the reliability and value of DHPs and ensure commercial success.
To help, DiMe collaborated with healthcare stakeholders to create Evidence DEFINED to define ‘what good looks like’ when it comes to evidence evaluation.
-
Offers payers, employers, health systems, and other stakeholder users a harmonized, rigorous, rapid approach to assessing the clinical value of DHPs
-
Underlies the evidence to help payers make quicker coverage decisions on DHPs being presented to them daily to ensure good market access for innovative health interventions
-
Helps DHP companies navigate their commercial strategy and demonstrate the value of their product to payer audiences
Meet the experts who developed the Evidence DEFINED framework
A group of 17 experts with different disciplinary backgrounds collaborated to develop the Evidence DEFINED framework. This sprint team represented experts from a variety of different work settings and multiple regulatory and geographic regions.
Meet the team:
We would love to hear how you have used, or plan to use, Evidence DEFINED to determine the reliability and value of your digital health products. Please share your story with us, and we’ll create a case study for you to showcase your leadership in this space!
Evidence DEFINED Resources
Quick Start Guide
A process overview for the Evidence DEFINED Framework
Download or share this resource:
Definitions Matter
Digital health products are patient-facing products that meet the criteria shown below. Use the table to help define the product you are using.
1. The product falls into one of the three classes of digital health technologies that were defined in a collaboration of stakeholders representing digital health trade organizations.
| Product Class | Product Class Definition |
|---|---|
|
Product Class:
Digital Health
|
Product Class Definition:
Digital health includes technologies, platforms, and systems that engage consumers for lifestyle, wellness, and health-related purposes; capture, store or transmit health data; and/or support life science and clinical operations.
|
|
Product Class:
Digital Medicine
|
Product Class Definition:
Digital medicine includes evidence-based software and/or hardware products that measure and/or intervene in the service of human health.
|
|
Product Class:
Digital Therapeutics
|
Product Class Definition:
Digital therapeutic (DTx) products deliver evidence-based therapeutic intervention to prevent, manage, or treat a medical disorder or disease.
|
2. The product is designed to change one or more health behaviors.
3. The value of the product to the evaluator is contingent on the degree to which it improves one or more health outcomes. These can include clinical outcomes (eg, incidence of diabetic retinopathy) or surrogate outcomes (eg, HbA1C).
Download or share this resource:
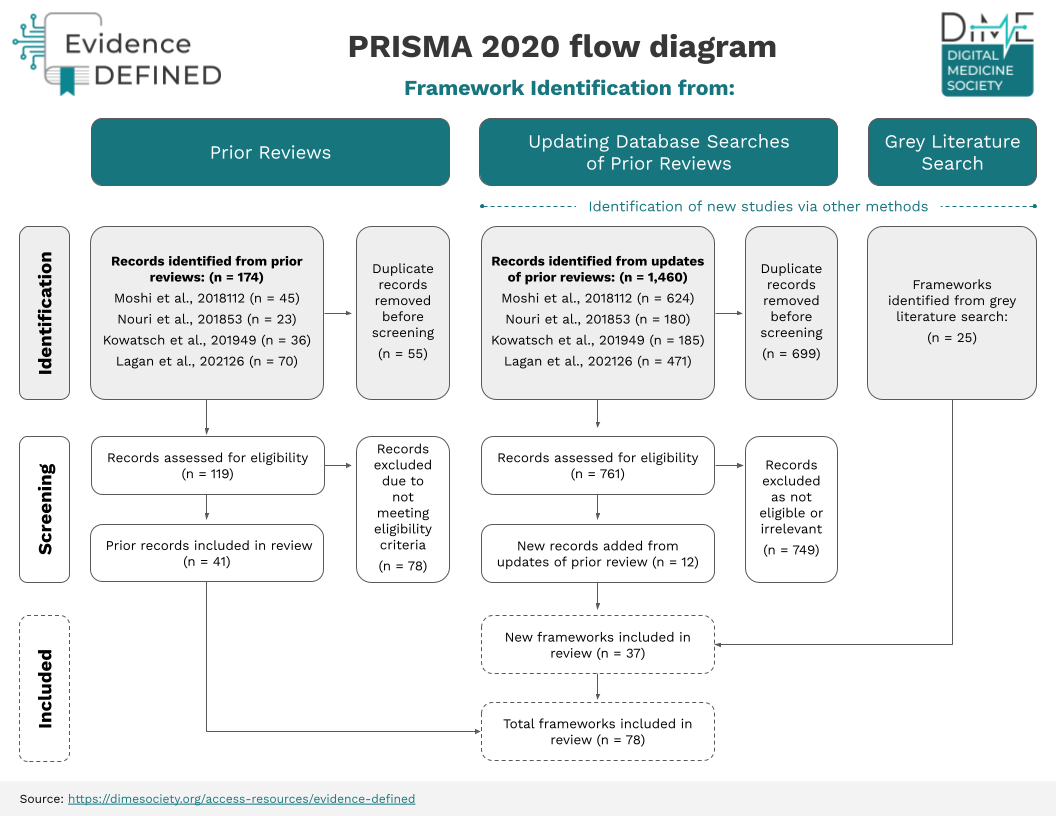
Analysis of Prior Evidence Assessment Frameworks
A total of 78 prior frameworks were identified as relevant by the Evidence DEFINED team. Prior frameworks are not sufficiently adapted to address evidence considerations unique to digital health, creating ambiguity for various stakeholders. This table includes frameworks that assess evidence alone, or evidence as well as other domains of assessment.
Download or share this Resource:
Checklist of Evidence Quality Criteria
Download or share this Resource:
Evidence-to-Recommendation Guidelines
| Actionability Level | Criteria | Adoption level that may be appropriate | Approx. enrollment that may be appropriate |
|---|---|---|---|
|
Actionability Level:
0 |
Criteria:
One or more of the following:
|
Adoption level that may be appropriate:
Adoption not recommended. |
Approx. enrollment that may be appropriate:
N/A |
|
Actionability Level:
1 |
Criteria:
All of the following:
|
Adoption level that may be appropriate:
Feasibility Pilot: Focus is on enrollment, engagement, user experience, safety.
|
Approx. enrollment that may be appropriate:
N ≤ ~100 |
|
Actionability Level:
2 |
Criteria:
All of the following:
Acceptable or likely acceptable (per GRADE) to stakeholders |
Adoption level that may be appropriate:
Small Clinical Pilot: Primary outcomes are clinical.
|
Approx. enrollment that may be appropriate:
Up to several hundred.
|
|
Actionability Level:
3 |
Criteria:
All of the following:
|
Adoption level that may be appropriate:
Large Clinical Pilot: Primary outcomes are clinical.
|
Approx. enrollment that may be appropriate:
~300 ≤ N ≤ ~3,000 |
|
Actionability Level:
4 |
Criteria:
All of the following:
|
Adoption level that may be appropriate:
May be appropriate to scale.
|
Approx. enrollment that may be appropriate:
No limit for appropriate patients.
|
Download or share this Resource:
Get Involved!
Catch the replay!
Watch as the co-authors showcased and discussed Evidence DEFINED during a DiMe Journal Club. Tune into the recording or access the presentation slides.
In this journal club, the co-authors came together to showcase the framework, which offers payers, employers, health systems, and other users a harmonized, rigorous, rapid approach to assessing the clinical value of DHPs and bringing them to market faster.
Engage in an upcoming project
If you would like to engage in DiMe’s upcoming IEP project and help streamline the path to regulatory and commercial success to optimize health outcomes for the greatest number of patients, learn more and share your interest to join: