Assessing the available options for measurement in the Alzheimer’s Disease and Related Dementia population
Identifying and selecting available technologies for any use case in health is complex. As we navigate the process, we often consider various questions about diagnosis, prognosis, or measurement. In the Alzheimer’s Disease and Related Dementia (ADRD) space, as with many neurocognitive disorders, there are additional levels of complexity given the blurred boundaries between disease presence and severity and between multiple or different dementia diagnoses. Choosing biomarker technology or electronic clinical outcomes assessment technology can be challenging, given the need for evidence in the particular population of interest.
Appraising technologies & assessing gaps
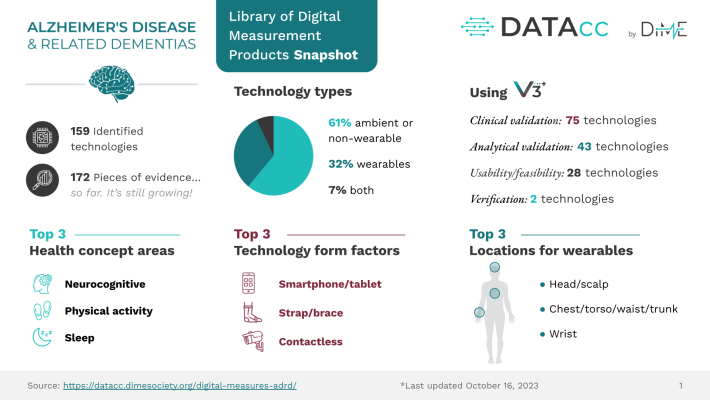
The Library of Digital Measurement Products for ADRD, developed by the Digital Health Measurement Collaborative Community (DATAcc) by the Digital Medicine Society (DiMe), is the start of a solution to speed up research and ease the use of digital health technology. A crowd-sourced resource, the library collates available evidence such as manuscripts, posters, and regulatory filing documents to help researchers access the data they need to identify and evaluate technologies required for their research. Users can filter the library by many fields, including health concepts, and identify the existing technologies and research in the ADRD population for that technology.
To assess a technology’s value in a specific use case and ultimately make informed decisions by relying on reported research, using resources such as DiMe’s EVIDENCE Checklist will ensure all necessary data is contained within the work. Locating technology-related evidence for assessment can be a challenging task. Research teams may be unaware of newer technologies, potentially causing them to overlook possible solutions.
Gathering supportive evidence
The value of a resource like this relies heavily on it being up-to-date. With cyclical literature review updates and submissions from the digital health community, the library will continue to grow and become increasingly valuable with each new update.
In addition to the digital health community, the research community must also produce high-quality evidence. In line with the EVIDENCE Checklist, researchers must specify, for example, the population used in any evidence they produce. Understanding the method for confirming diagnosis is equally important for those wanting to have faith in the technology’s analytical and clinical validation and its usability and applicability to the group under study.
The use of evidence must be approached responsibly. In alignment with the argument-based validity approach, a measure – in this case, a digital health technology measure – is never considered to be a validated measure. Instead, evidence for validity needs to be built up to support the use of a measure for a specific concept of interest within a specified context of use. Users must evaluate evidence in the library concerning their particular research question and understand the gaps that they may need to address.
With high-quality research kept in a current, up-to-date compendium, which displays the information users need in a highly manipulatable way, we can speed up digital health research and development. The Library of Digital Measurement Products, currently for ADRD research, is the bold first step in this process. Stay tuned for how this library will continue to expand with digital health measurement products in other research areas at DATAcc.
View the library here.
Do you have a technology or additional evidence to add to the library? Contribute here.
DATAcc aims to improve health outcomes, health economics, and health equity as a collaborative community with the FDA’s Center for Devices and Radiological Health. Get involved in our efforts!



