This course is now free!
Online Course
Applied Digital Health Ethics
Take your next step to ensure that the field of digital health is worthy of our trust.

I found this course has given a very detailed overview of ethics by design approach. It definitely helped me to be more conscious in my work including planning of AI/ML healthtech projects with considerations to ethics as taught here.

Course overview
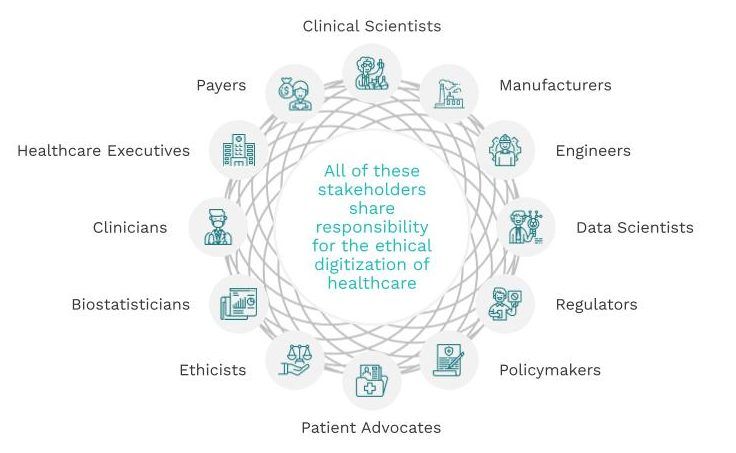
Ethics is foundational to a digital health industry that helps, not harms. Every one of us working in the field ╴must have foundational knowledge in ethics to ensure that we realize the full promise of the digitization of healthcare to improve lives, for everyone.
Learning outcomes
Prepare to apply an ethical approach to ensure that the benefits of digital health outweigh the risks of harm, for all people. Identify the risks and define mitigation strategies associated with artificial intelligence, and machine learning methodologies in healthcare and research. Explore the relationship between ethics and equity in the digital era of healthcare.
Discover how ethical approaches can build public trust in digital health.
Topics include
-

Ethics by Design
Defined with a framework from which to build a culture of ethics.
-

Ethics and Tech
Working with digital data and artificial intelligence (AI) ethically.
-

Ethics and Equity
Fairly applying ethics to build trust.
Meet the instructors
Learn from DiMe experts and world class guest faculty – including patient experts, applied ethicists from industry, and academia – at the cutting edge of the most difficult ethical questions in digital health.
Reviews
Read what our learners are saying…

I use it to help steer the teams into ethical software development.

I found this course has given a very detailed overview of ethics by design approach. It definitely helped me to be more conscious in my work including planning of AI/ML healthtech projects with considerations to ethics as taught here.

This outstanding and practical course helps to reflect on the importance of ethics when working on digital health and demonstrates to you the necessity of being an active contributor.

This course provided tangible, real-world examples of ethics in action to guide my every day decision making in building digital health solutions. I especially appreciated the video content featuring thought leaders articulating how they demonstrably apply these core principles in their specific areas of expertise.

A good compilation of current issues and guidelines. “applied digital health ethics.” Completed the course today.

The program provided a comprehensive understanding of ethical considerations in healthcare technology and equipped me to navigate the digital landscape with confidence and integrity. The instructors were experts and the curriculum covered a wide range of topics. I highly recommend this course to any healthcare professional looking to deepen their knowledge in digital ethics. Thank you, DiMe, for such a valuable learning experience.

“I gained a better understanding of the ethics associated with providing useful digital health solutions to patients. I will be more diligent and specific in communications with partners and vendors.”

“The course provides great foundational awareness to incorporate ethics into every decision making step in the process of bringing a device to market. E.g., as a design input requirement.”

“I gained a better understanding of the ethics associated with providing useful digital health solutions to patients while still protecting them. As a result of taking this course, I will be more diligent and specific in communications with partners and vendors.”

“This course gave me additional insights that I can apply and share with our clients.”

More courses coming soon
The Digital Medicine Academy®️ is committed to offering valuable lessons in pace with the evolving digital healthcare field. Check back for new course offerings.