
Dear White House: How to Build the Future of the U.S. Clinical Trials Ecosystem with Digital Health Tools
In January 2023, DiMe submitted a response to a Request for Information published by the White House Office of Science and Technology Policy (OSTP). Here, we share insights from that response and outline what we believe to be a path forward for digitally-enabled clinical trials.
It took an unprecedented global pandemic to bring attention and action to weaknesses in the clinical evidence generation system, from cracks in the data collection infrastructure to grave health inequities facing many communities.
To address the acute data collection needs of the COVID public health emergency, COVID clinical trials were initiated at a previously unimaginable pace, with more than 2,024 trials activated between the beginning of the pandemic and February 2021. Unfortunately, except for the success of large-scale coordinated evidence-generation efforts such as ACTIV (U.S.) and RECOVERY (UK), the overall global research response was inefficient and fragmented, characterized mostly by trials that were not designed to yield actionable evidence or deliver sufficient enrollment rates. Further thousands of trials—around 80% of non-COVID-19 trials—were stopped or interrupted primarily due to reliance on in-person research visits and drug distribution, leading to further delays in life-saving therapies going to the market.
Challenges in the response of the U.S. clinical trials infrastructure to COVID included issues in:
- Activating clinical trial sites quickly enough to keep pace with rolling surges in COVID rates across the country
- Recruiting and enrolling a sufficient volume and diversity of participants in COVID research
- Providing resources to equitably address the trial participation needs of underserved communities
- Aiding healthcare providers in carving out time to collect data on investigational medical products during the provision of emergency care
- Comparing the results of multiple ongoing trials because of discrepancies in clinical outcome measures and standards for data collection
- Collecting and leveraging real-world data (RWD) to inform real-time decision-making and an efficient public health response
These challenges also impacted the way evidence was generated for other conditions outside of the public health emergency context, indicating pervasive problems in our clinical trial infrastructure and providing the basis for broad clinical trial improvement after the public health emergency ends this May.
The now: Building a formidable foundation for future research
A resilient and efficient clinical trial infrastructure is foundational today to identify effective vaccines, therapies, and other interventions needed to support high-quality care delivery as well as an effective response to all future global public health emergencies. But innovation in clinical research has not kept pace with the evidence needed to support broad transformation in the way we deliver care.
The equitable and effective implementation of digital medical technologies to support patient screening, trial enrollment, clinical data collection, and real-world monitoring for product safety and effectiveness can modernize our clinical research enterprise and help us to better meet the decision-making needs of clinical, regulatory, and payer stakeholders. Digital tools such as platforms/solutions for clinical trial management, virtual visit/telehealth tools, screening tools powered by artificial intelligence, and digital clinical and non-clinical measures can increase access to clinical research and expedite trial conduct.
These tools are helpful across the treatment lifecycle and can support:
1. Trial sites where evidence generation is less costly and better integrated with routine care
The ability to automate and integrate aspects of clinical research with routine care delivery is critical to improving the evidence-generation capacity and representative nature of the clinical trials enterprise. Increased investment in data collection infrastructure (i.e. platforms and standards) and support for the adoption of turn-key clinical trial management software that allows for the automatic transfer of patient health record data to fields in electronic data capture systems is especially supportive of research that fits in with care delivery workflows. The development of appropriate incentives for research participation, health system-level support for technology adoption, and a plan for workforce training can ensure consistent quality in data collection and expedite trial completion. Such work can simplify and automate trial participation, making it more feasible for health systems and providers to be included in evidence-generation efforts, therefore yielding more generalizable and actionable results.
2. Decentralized research that expands trial access and speeds up time to results
Digital research platforms that enable telehealth visits, simplified eConsent, and remote data collection can extend clinical trial access to a larger and more diverse group of patients. Such platforms can also reduce the administrative burden of participation in research, precluding the need to navigate complex consent and data collection processes as well as the need to travel for research visits.
Sponsors of research should consider how to effectively leverage digital research platforms for remote data acquisition to make it easier to enroll harder-to-reach patients and supplement the capacity of site-based data collection during evidence-generation efforts.
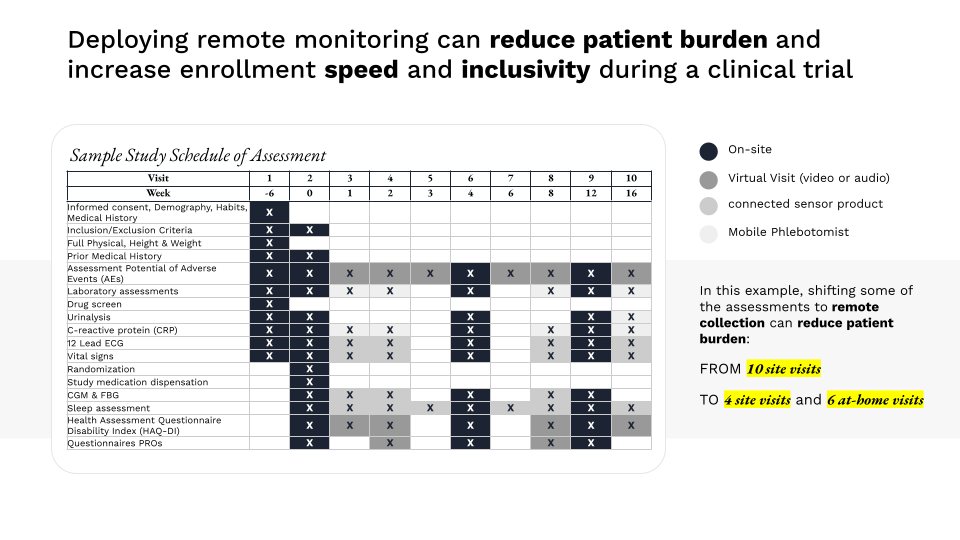
Image: The Playbook: Digital Clinical Measures
3. Efficient trial matching, increased trial enrollment, and the delivery of precision medicine
Artificial intelligence (AI)/machine learning (ML)-enabled screening software can accelerate understanding of disease progression to support protocol design and cohort selection. Systematic research efforts to assess the performance of such software – including the development of criteria for the ethical, effective, equitable, and safe use of AI/ML and the data sets they mine – are essential to ensuring that they reduce and do not perpetuate bias and can optimally support clinical trial conduct.
Other tools that simplify and expedite trial enrollment include eConsent platforms, which have tremendous potential for:
- Supporting patients to navigate and consent to participate in clinical trials where efficiency of enrollment and decreased patient burden to support retention matters a great deal
- Reducing administrative burden of enrollment as well as enrollment-related protocol deviations, supporting increased trial participation, and speeding up time to results
- Promoting health equity, allowing for approaches that ameliorate disparities in health literacy (i.e. through the use of videos, etc.) that impact trial enrollment
- Designing seamless consent processes so patients can consent to the use and reuse of their data, as well as participate in trials with innovative designs, is important for enhancing the evidence-generation capacity of the clinical trials enterprise as a whole.
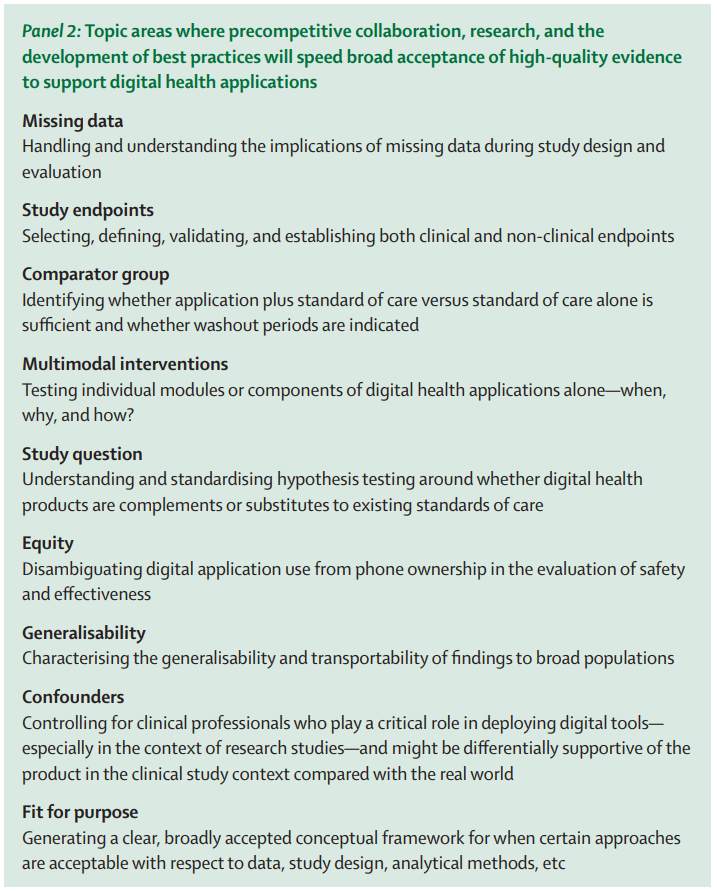
Image: Advancing digital health applications: priorities for innovation in real-world evidence generation
4. The use of RWE to inform decision-making
Connected digital technologies that support patient monitoring and remote data collection can allow us to extract insights from the wealth of continuously collected health data generated during the course of routine care delivery. To enhance our ability to leverage real-time learnings from RWD generated using sensor-based technologies, we must develop guidelines, standards, and recommendations to address real and perceived deficits in the quality of real-world data and increase trust in sensor-based products. Work to increase the quality and acceptance of real-world evidence (RWE), outlined to the right, would address important issues that can help unlock the promise of flows of RWD for driving faster and better decisions across the healthcare
The future: Digitally-enabled clinical trials for faster and more actionable results
The rapidly evolving state of digital health technologies provides the opportunity for new and innovative mechanisms to improve access and efficiencies across the clinical trials ecosystem. Appropriately leveraging digital tools to better integrate medical product development and biomedical research into routine clinical care will improve our collective ability to respond to viral public health emergencies, as well as to the public health crises of health inequity, healthcare costs, and declining life expectancy.
For additional details on approaches to the implementation of digital health technologies to support the development of a more robust, sustainable, and equitable trial infrastructure:
- Read DiMe’s full response to the OSTP’s recent Request for Information.
- Develop a strategy to use specific digital tools to address challenges facing the clinical trials industry while advancing health equity. Implement resources from DiMe’s Diversity, Equity, and Inclusion in Digitized Clinical Trials project to support efforts for more diverse, equitable, and inclusive clinical trials.
