
Navigating CMS’s Innovative Coverage Pathway for Digital Health Products
The Medicare coverage process for new digital health products has been a subject of ongoing debate. For years, digital health solutions have voiced concerns about the lack of clarity, slow pace, and numerous obstacles they face when seeking reimbursement. These challenges impede innovation and hinder progress within the industry. While the newly proposed CMS coverage pathway, Transitional Coverage for Emerging Technologies (TCET), holds promise as a step in the right direction to align regulatory and payer decision-makers, the industry is vastly divided over the proposed reimbursement pathway aimed at addressing product coverage challenges, thereby impacting timely and predictable access to innovative digital health products. The TCET pathway uses national coverage determination (NCD) and coverage with evidence development (CED) processes with the aim to:
- Expedite Medicare coverage of certain FDA-authorized Breakthrough Devices
- Reduce uncertainty about coverage options through a premarket evaluation of potential harms and benefits of technologies while identifying any important evidence gaps
- Help coordinate benefit category determination, coding, and payment reviews
- Allow manufacturers to address any evidence gaps through fit-for-purpose studies
The quest for clarity and timeliness: a second attempt
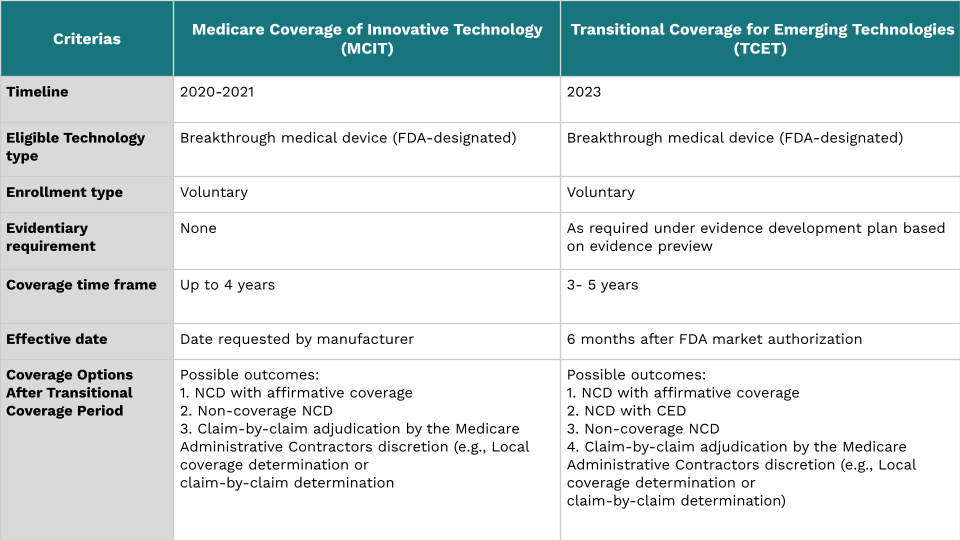
Following the Medicare Coverage of Innovative Technology (MCIT) rule in 2021 by the Trump Administration, there was renewed optimism for a guaranteed four years of Medicare coverage for FDA-approved breakthrough devices via accelerated coverage. However, concerns were raised regarding the limited evidence supporting device benefits and risks for Medicare patients that ultimately led to the sunsetting of the MCIT pathway. However, efforts to enhance the coverage process persisted. CMS adjusted its approach (Image 1) to focus on device scrutiny, assuring stakeholders that an alternative would be developed to streamline coverage for innovative devices while prioritizing safe and high-quality care.
Exploring the TCET pathway for coverage
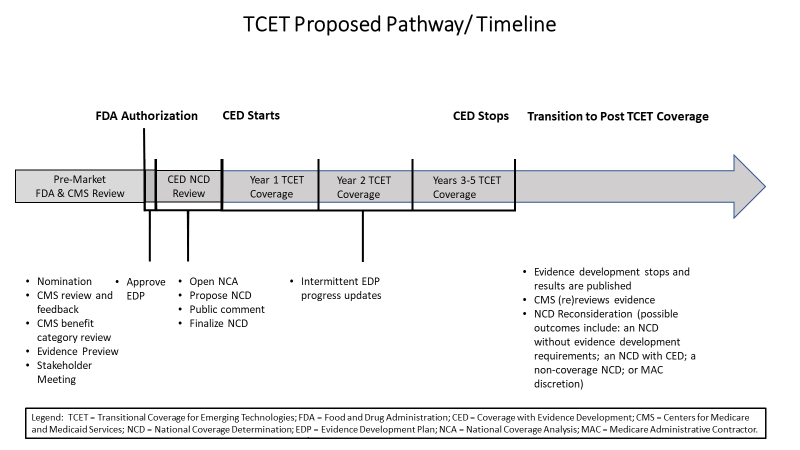
The TCET pathway utilizes existing NCD and CED processes to expedite Medicare coverage for certain Breakthrough Devices. It’s designed to reduce uncertainty by evaluating these technologies’ potential benefits and harms before they enter the market while identifying any evidence gaps. Manufacturers are offered increased pre-market engagement opportunities and flexibility in addressing these gaps through fit-for-purpose studies. Such a design is to balance multiple considerations when making coverage determinations. As illustrated in the image below, the pathway follows a structured timeline to ensure efficient and transparent processes.
Eligibility, evidence plan, and pathway transition
Given the unique considerations, appropriate candidates for the TCET pathway would include medical devices that are:
- Certain FDA-designated Breakthrough Devices;
- Determined to be within a Medicare benefit category;
- Not already the subject of an existing Medicare NCD; and
- Not otherwise excluded from coverage through law or regulation.
Evidence development is a critical aspect of the TCET pathway. CMS recognizes the significance of reliable evidence and collaborates with manufacturers to address any identified gaps through the creation of an Evidence Development Plan (EDP). This plan allows manufacturers to design appropriate studies, either traditional or fit-for-purpose, to address these gaps. Collaboration between CMS, manufacturers, and AHRQ ensures scientific integrity and relevance to the Medicare population, minimizing duplicative or conflicting evidence requirements.
The TCET pathway follows a well-defined timeline to ensure transparency and efficiency. After a device is accepted into the pathway and receives FDA authorization, CMS initiates the NCD process. CMS aims to finalize a TCET NCD within six months of FDA authorization, providing temporary coverage tied to evidence generation. Post-TCET coverage involves an updated evidence review conducted by a third-party contractor. This systematic literature review and assessment against benchmarks and quality measures inform CMS’s proposal for an NCD without evidence development requirements, an NCD with continued evidence development requirements, a non-coverage NCD, or a rescission of the NCD. Overall, the TCET pathway establishes robust mechanisms for evidence generation, timely decision-making, and ongoing evaluation to ensure the provision of high-quality, patient-centered care.
The future of CMS’ pathway: Implications for industry
With overwhelming positive interest in the TCET from the industry, there seems to be a common underlying industry-wide concern that the program may have a similar fate to the parallel review program: a joint FDA-CMS pathway that allows agencies to review devices and hand down coverage decisions, at the same time to streamline the evidence-generation process. The parallel review program started with a lot of excitement. However, only two devices have successfully gone through it.
Adding to the complexity, a backlog of unapproved breakthrough devices raises questions about Medicare’s capacity constraints to handle the influx of potential applications to review and collaborate. CMS anticipates a restricted number of devices being selected and endorsed (8 nominations and approving a maximum of 5 candidates) annually, prioritizing devices that have the potential to provide substantial benefits to a large number of Medicare beneficiaries. Despite the expedited pathway, the TCET program’s impact may be limited because, while dozens of devices receive breakthrough designation annually, CMS expects a smaller number of TCET nominations and maintains certain eligibility requirements, such as the “benefit category” criteria, which may exclude certain innovative device types from Medicare coverage. Additionally, concerns were raised regarding the impact of lengthy reimbursement delays on companies’ willingness to develop innovative technologies. Nevertheless, the industry views these changes as a positive step in the right direction.
Next steps for the industry: Take action
Even though access to new medical technologies is crucial for improving patient care and promoting innovation in healthcare, and while there is a consensus among industry groups and policymakers that the existing process is inefficient and unpredictable, there is a need to strike a balance between expediting access to innovative technologies and ensuring robust evidence generation. As CMS works on finalizing the proposed notice, you can:
- Lookout for upcoming activities and documents by CMS that includes detailed fit-for-purpose guidance document, NCD pilot program, CMS guide for medical technology companies and other interested parties, and future guidance documents to review meaningful health outcomes in other priority therapeutic areas.
- Make your thoughts on TCET heard before August 26, 2023, on the TCET notice and proposed CED and Evidence Review guidance documents. Use DiMe’s federal comment toolkit to submit your comments.



