
Chapter 2 – Section 3
A human-centered development approach

A human-centered development approach ensures that children and their care systems are placed at the center of product development and that all of their needs and perspectives are taken into account.
This section emphasizes the importance of centralizing users and embedding clinical expertise into your product development process to build engaging, relevant, and clinically impactful tools.

Key insights
Guiding product development with evidence: A call from the clinicians on our Playbook team
Developing DHTs that truly meet the needs of pediatric patients requires more than innovative ideas and feedback from users; it demands clinical expertise be embedded in product teams. Embedding these clinical subject matter experts (SMEs) from the onset of product ideation and conceptualization will help you develop technology that actually drives outcomes and improves patient lives.
Clinical SMEs help your product teams:
-
 Ensure evidence-based practices: Clinicians can provide insights grounded in the latest research and clinical guidelines, enhancing the credibility and effectiveness of DHTs.
Ensure evidence-based practices: Clinicians can provide insights grounded in the latest research and clinical guidelines, enhancing the credibility and effectiveness of DHTs. -
 Align with real-world needs: Clinicians understand the complexities of pediatric care that may not be so obvious to technical and business-oriented teams, enabling them to:
Align with real-world needs: Clinicians understand the complexities of pediatric care that may not be so obvious to technical and business-oriented teams, enabling them to:- Prevent costly rework and design flaws.
- Ensure patient safety and regulatory compliance.
- Build credibility with healthcare stakeholders to accelerate adoption.
-
 Drive improved outcomes: By working together, we can develop solutions that not only address clinical challenges but also lead to measurable improvements in patient health.
Drive improved outcomes: By working together, we can develop solutions that not only address clinical challenges but also lead to measurable improvements in patient health.
From clinical expertise to human-centered design
While clinical SMEs are integral to an evidence-based understanding of pediatric health needs and opportunities for meaningful impact, there is an additional layer of engagement needed to ensure that patients and caregivers are adequately incorporated throughout a product’s lifecycle. Let’s delve deeper into how we can truly meet the needs of pediatric patients, their caregivers, and the clinicians that deliver their care through the lens of human-centered design and user research.
Fusing user research with clinical expertise
While clinical SMEs are essential members of product teams, qualitative and quantitative insights from your end users (patients, caregivers, and clinicians) should also guide your decision-making throughout the product development lifecycle. Both user research and clinical SMEs are separate, but integral, sources of product design guidance.
Explore further by hovering over the (+) sign.
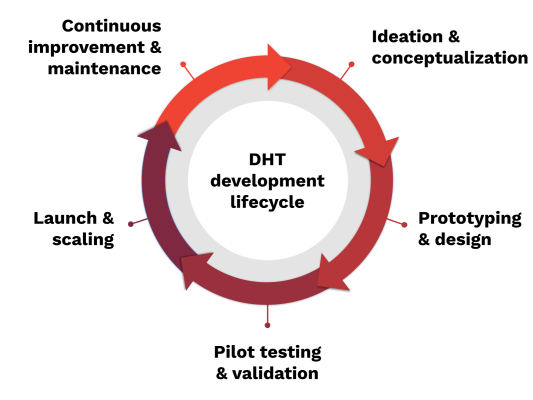
Ideation and Conceptualization
Primary Stakeholders: Children, Caregivers, Clinical Users
Key Activities:
- Identify Real Needs: Gather detailed insights from children and caregivers about unmet health needs and preferences. Consult clinical users for their perspective on care challenges.
- Early Input on Solutions: Collaborate with clinicians, families, and children (when appropriate) to test preliminary ideas and ensure solutions align with lived experiences.
- Supporting Stakeholders: Product Designers, Researchers, Compliance Advisors
- Key Information to Gather: Caregiver experiences on what they struggle with daily. Clinician perspectives on gaps in pediatric care and potential digital health solutions.
Prototyping and Design
Primary Stakeholders: Children, Caregivers, Clinical Users
Key Activities:
- Co-Design for Usability: Engage children (based on age-appropriateness), caregivers, and clinical users to co-design prototypes, ensuring the technology is user-friendly and accessible.
- Privacy and Safety Checks: Involve compliance advisors in the design process to prioritize children’s privacy and ensure data safety measures meet pediatric needs.
- Supporting Stakeholders: UX and Product Designers, Data Scientists
- Key Information to Gather: Feedback from caregivers on the ease of use and potential challenges. Clinical user input on how the technology can best fit into care workflows.
Pilot Testing and Validation
Primary Stakeholders: Children, Caregivers, Clinical Users
Key Activities:
- Real-World Testing: Pilot the technology with children and families in real-life environments. Clinical users provide hands-on insights into the functionality and usability in care settings.
- Assess Efficacy and Safety: Collect data on efficacy and safety from the clinical environment and receive feedback on pediatric-specific needs.
- Supporting Stakeholders: Clinical Trial Teams, Regulatory Advisors
- Key Information to Gather: Performance data from clinical and home settings. Direct feedback from children, when possible, on comfort and ease of use, as well as caregiver perspectives on day-to-day use.
Launch and Scaling
Primary Stakeholders: Children, Caregivers, Clinical Users
Key Activities:
- Implementation in Care Settings: Ensure clinicians and caregivers have the resources and support needed for smooth onboarding.
- Patient Education and Support: Offer training resources to families and caregivers for optimal use, and gather feedback on initial use experiences.
- Supporting Stakeholders: Healthcare Administrators, Regulatory Bodies, Insurers
- Key Information to Gather: Caregiver and clinician feedback on challenges with technology adoption. Data on usage patterns and any support needs in diverse settings.
Continuous Improvement and Maintenance
Primary Stakeholders: Children, Caregivers, Clinical Users
Key Activities:
- Regular Check-Ins and Updates: Periodically engage children, caregivers, and clinicians to identify needed improvements and assess the ongoing impact on health outcomes.
- Feedback Loop for Enhancements: Use input from real-world usage to guide software updates and feature enhancements to address evolving needs.
- Supporting Stakeholders: Product and Customer Support Teams, Data Analysts
- Key Information to Gather: Long-term feedback from caregivers and clinical users on usability, durability, and effectiveness. User-reported issues and requests for new features.
Read DiMe’s Framework for Inclusive Development of digital measures.
The FDA’s Division of Patient-Centered Development can help you understand how to integrate the patient perspective into the total product life cycle of medical devices.

Key insights
Meaningful engagement: A crossfunctional framework for digital therapeutics
Clinical science should always guide digital health product development. However, simply building a clinically relevant tool will not suffice. You must also consider the human interaction and engagement of your technology and understand how to optimize engagement amongst users with the most clinically relevant features.
The authors of this article outline a 4-step evidence-based approach to including users in the development of a digital therapeutic for adolescents with depression. The authors emphasize:
- User-centric design (UCD): Integrating patients’ perspectives throughout the design process leads to digital therapeutics (DTx) that are more engaging and relevant to real-world needs.
- Collaborative development: Involving clinical SMEs and users alongside developers allows for DTx that better fit into actual healthcare settings and meet patients’ everyday challenges.
- Iterative feedback: Continuous user feedback enables adaptations that improve usability, fostering sustained engagement and maximizing the therapeutic impact.
Digital Therapeutics (DTx):
Evidence-based therapeutic interventions delivered through software programs to prevent, manage, or treat medical conditions. Typically used independently or alongside medications, devices, or other therapies. Can offer personalized and accessible healthcare solutions. Key characteristics include their ability to deliver measurable clinical outcomes and their integration with clinical practice to support patient care.
How can human-centered design and user research help serve children medically?
Human centered design (HCD) is an approach to interactive systems that aims to make systems usable and useful by focusing on the needs and requirements of users. HCD focuses on all individuals interacting with the system, including direct users, stakeholders, and in the broader context of human interaction. HCD provides a framework for continuously involving children and other end users in research and development to create digital health tools that are both engaging and impactful, ensuring the tools resonate with their real-life experiences.
User research is a foundational mechanism of HCD that delivers the insights, feedback, and validation needed to create products that are effective, safe, and enjoyable to use. This research is in addition to clinical SME involvement. The Playbook team has created a series of tips and considerations when centering the child and care system in your product development process.
In order to understand what matters to the end users, you must include them in your product development process.
Human centered design (HCD):
An approach to interactive systems that aims to make systems usable and useful by focusing on the users, their needs and requirements, and by applying human factors and usability knowledge and techniques.
User research:
A foundational mechanism of human-centered design (HCD) that delivers the insights, feedback, and validation needed to create products that are effective, safe, and enjoyable to use. This research is in addition to clinical SME involvement.
HCD and user research in practice
The maker of Amir’s mobile app considered the entire care system when designing it, taking into account the needs and expectations of caregivers, healthcare providers, and other individuals. A clinical SME was embedded in the product team to ensure the tool’s clinical relevance and guide the development process.
User research helped gather additional insights directly from target users – through interviews, observations, and testing – to inform the design process. This holistic approach helps ensure that the app is both user-centered and clinically relevant:
- Supports communication between Amir and his care team = fewer flare ups
- Integrates with clinical workflows = Dr. Johnson can actually adopt this tool in her busy daily practice
- Addresses the logistical needs of his caregivers and doctors = enhanced care coordination and peace of mind

Meet Amir
Amir is a 10-year-old African American boy living in a low-income neighborhood in New York City. He has struggled with asthma since he was 7, often experiencing flare-ups triggered by environmental factors such as mold and dust in his small apartment. His mother, Tasha, and father, Bryce, both work full-time and frequently miss signs of Amir’s worsening condition, leading to many unplanned hospital visits, missed school days for Amir, and missed workdays for Tasha and Bryce.
Now, Amir’s pediatrician has introduced a smart inhaler connected to a remote monitoring platform. This inhaler tracks his usage and provides real-time data on his condition, which is shared with Tasha and Bryce and Amir’s healthcare provider. Since adopting this DHT, Amir’s asthma has become much more manageable, significantly reducing emergency room visits and improving his overall quality of life.
Personas illustrate key points in action, showcasing real-world applications and practical scenarios based on fictional personas.

Meet Tasha and Bryce, Amir’s caregivers
Tasha and Bryce have two children and both work full-time. Their son, Amir, has struggled with asthma since he was 7, and managing his condition has been a constant source of stress for both parents.
Since Amir’s pediatrician introduced a smart inhaler, Tasha and Bryce are able to be more engaged in his care. The platform tracks Amir’s inhaler use and symptoms in real time, allowing them to monitor his condition even while at work. This DHT has given Tasha and Bryce more control and confidence in managing Amir’s asthma, significantly reducing their stress, frequency of emergency visits, and helping them balance caregiving with their jobs.

Meet Dr. Johnson, Amir’s pediatrician
Dr. Johnson is a pediatrician practicing in a community clinic in New York City. She has been caring for Amir and other children with chronic conditions like asthma for several years. A busy schedule and a large community to serve make it difficult for Dr. Johnson to provide consistent, timely interventions. She often only sees patients when their conditions have already worsened, and she can hardly keep up.
With the introduction of remote monitoring tools like smart inhalers and wearables, Dr. Johnson can now track her patients’ health data between visits. The real-time information allows her to make informed decisions about treatment plans and to intervene before issues escalate. These digital tools have streamlined her workflow, enabling Dr. Johnson to enhance the quality of care she provides while improving outcomes through timely, data-driven interventions.

Meet Ryan, Amir’s school nurse
Ryan is a school nurse working in a public school in New York City, overseeing the health of hundreds of students, including Amir, who has asthma. Before the implementation of digital health technology, Ryan relied on sporadic updates from parents and teachers, often learning about a student’s health issues only during emergencies.
Now, with the integration of digital health data from Amir’s smart inhaler, he receives timely alerts regarding his condition. This capability allows him to proactively manage Amir’s care and prevent asthma attacks at school. The technology has made it easier for Ryan to oversee the health of students with chronic conditions, ensuring early intervention and improved health outcomes.
Recruiting participants for user research

- Identify the target users: Select users (children, caregivers, and clinicians) who represent the real-world audience of the product.
- Develop a diverse recruitment strategy across demographics, including ethnicity, socioeconomic status, and geographical location.
- Virtual recruitment platforms like Savvy Cooperative and User Interviews can help you reach young people and caregivers.
- Collaborate with pediatric and community healthcare providers who can refer patients. They can help identify families willing to participate and can explain the value of the research to them.
- Engage with patient advocates and advocacy groups focused on specific health conditions to reach families directly involved with those issues.
Ethics and compliance considerations in user research

- Choose the right consent and assent structure: Implement a clear and informative consent process for caregivers and assent for children.
- Follow state and federal guidelines for parental consent and child assent.
- Clarify how parents and children will be informed about data usage and set clear expectations for the involvement.
- Comply with regulatory guidelines that protect children’s data and privacy, like HIPAA and COPPA. Understand the legal requirements around collecting and handling health data for minors, such as when you need approval from an independent Institutional Review Board (IRB). Read more about child consent from these authoritative sources: The Common Rule 45 CFR 46; Office for Human Research Protections (OHRP).
- Ensure accessibility of your consent forms by writing them at a seventh grade reading level, and providing documents and information in multiple languages.
User research and research design

- Account for children’s developmental age: Tailor questions and tasks to match the cognitive and emotional development of the child. For younger children, use simple language and interactive tasks to gather insights.
- Choosing the right research methods: Select methods like interviews, focus groups, or usability tests that match the developmental stage of the children and the needs of the caregivers. Ensure that research activities are age-appropriate and not overly burdensome.
- Offer an accessible research environment: Consider whether your target participants have access to the place of research. If virtual, ensure proper access to computers and other technology needed to participate.
- Establish appropriate outcome metric targets: Define metrics that align with children’s developmental stages, health needs, and product features, ensuring they are meaningful and achievable.
Informed Consent:
Process that requires a potential trial participant be given all of the information needed to make a sound decision about whether to volunteer to willingly participate. For informed consent to be meaningful, participants need to be “tech-literate” enough to understand the specifics of how their data will be obtained and used, or they need to be appropriately supported to understand these specifics.
Assent:
A child’s affirmative agreement to participate in a clinical investigation. Assent must be sought in addition to the consent of a legally authorized representative or surrogate when the individual is sufficiently cognitively capable of understanding the nature of his or her participation in a research study.

Key insights
Consenting children and caregivers into formative research
DiMe’s Digital Measures: Nocturnal Scratch project conducted qualitative interviews with child-caregiver dyads to identify meaningful aspects of health (MAH) connected to the symptom of nocturnal scratching in patients with atopic dermatitis. The project provides helpful examples of ethical and compliant consent and assent structures for children and their caregivers being involved in early exploratory research. Consider using the templates linked below for your own research.
Child’s Assent to Participate in a Research Study
Accounting for age and development in user research
As you’ll remember from earlier in this microplaybook, children’s needs change considerably over time and these nuances must be considered when including them in user research. The Playbook team presents three categories of child and caregiver relationships and explores how recruitment, ethical considerations, and research methods might change accordingly.
Caregivers as the main health manager
 Maya, is invited to participate in a study evaluating a new wearable technology. Her parents, Isabel and Marco, provide consent through a recruitment platform, and Maya gives verbal assent with their guidance. During an in-person session, researchers use age-appropriate methods like playful observation and usability tests to gather her feedback on the tool’s interface.
Maya, is invited to participate in a study evaluating a new wearable technology. Her parents, Isabel and Marco, provide consent through a recruitment platform, and Maya gives verbal assent with their guidance. During an in-person session, researchers use age-appropriate methods like playful observation and usability tests to gather her feedback on the tool’s interface.
Recruiting participants. Maya’s parents were told about this opportunity by her doctor. Because she is so young (3 years), the researchers decided it would be valuable to include one of Maya’s parents in the research as well.
Consent and assent. Maya must provide her written or verbal assent to participate after one of her caregivers has fully consented to their participation.
Research design. The researchers in this case chose to conduct in-person research given how young Maya is and the impracticality of her participating virtually. They conducted a drawing activity to facilitate the conversation with Maya and interviewed her caregivers to gain more insights into her care needs and expectations.
Take home: Consider the setting in which you engage with users and age-appropriate methods (e.g. observational studies for infants, guided usability tests for a 10 year old).
Children begin gaining autonomy

 Amir and his parents are participating in a study to assess the usability of a mobile app to manage his asthma. His parents consent to his involvement, and Amir provides written assent after the study details are explained in a way he understands. In a virtual session, researchers use a combination of interactive usability tests and short interviews to capture Amir’s feedback on the app’s features and ease of use.
Amir and his parents are participating in a study to assess the usability of a mobile app to manage his asthma. His parents consent to his involvement, and Amir provides written assent after the study details are explained in a way he understands. In a virtual session, researchers use a combination of interactive usability tests and short interviews to capture Amir’s feedback on the app’s features and ease of use.
Recruiting participants. Amir’s parents were notified of the opportunity to participate in user research from his pediatrician, Dr. Johnson. As children like Amir become increasingly autonomous and familiar with technology in this category, they can lend real insight into content, usability and integration into daily life. At this age, he still relies on his parents considerably, and their input into the app is crucial.
Consent and assent. Amir must provide his written or verbal assent to participate after one or both of his caregivers has fully consented to their participation.
Research design. Amir already has unfiltered access to devices like computers, smartphones, and tablets between home and school and is fully capable of participating in a virtual research session. Both he and his parents attend an interview with a product designer to share more about their obstacles and expectations for getting Amir the best care possible.
Teens gain full autonomy over care decisions
 Parker was recruited for a UX testing session of a wearable that monitors his muscle function and daily activity levels. The goal of the research is to better understand how Parker uses the tool throughout his day. During a virtual enrollment session, his parents provided consent and Parker gave his assent. Researchers conducted a focus group to gather teenagers’ feedback on how the wearable supports their health tracking and whether its design meets their accessibility needs.
Parker was recruited for a UX testing session of a wearable that monitors his muscle function and daily activity levels. The goal of the research is to better understand how Parker uses the tool throughout his day. During a virtual enrollment session, his parents provided consent and Parker gave his assent. Researchers conducted a focus group to gather teenagers’ feedback on how the wearable supports their health tracking and whether its design meets their accessibility needs.
Recruiting participants. Parker saw an advertisement for this research on Instagram and registered his interest on a HIPAA-compliant online form.
Consent and assent. Because he was under 18, the researchers required the consent of one or both of his parents. Parker also had to provide written assent to participate. Given his age, the researchers asked if Parker would like his parents to be included in the research – he declined.
Research design. Parker agreed to participate in multiple research sessions. He first participated in a two-week diary study, observing and recording his experiences with the devices over the course of the day via a mobile app. The second research session was a focus group where he learned about his peers’ experiences and shared his own.
Key considerations:
Children grow & develop in varied ways. Their cognitive and physical development can take many different paths and the care system in place is always changing. These are some of the things that you should consider when building tools for them:
- Developmental age-appropriateness and relevance of content.
- The preferred language of the child.
- Gamification and engagement elements.
- Form factor of wearable devices.
- Accessibility for children with disabilities or special needs.
- Technology access and affordability.
- Availability of the right infrastructure (e.g., internet).
- Compatibility with assistive technologies.
- Caregiver involvement and access controls that account for the dynamic nature of these relationships.
Caregivers are uniquely positioned in the care of children. They are the primary facilitators of care until children become old enough to make their own healthcare decisions, and often maintain an “advisory” status as children become adults.
- Maintain a strong relationship with the healthcare provider. Consider how to harness the adjunct power of your DHT while centralizing this relationship.
- Support caregivers with different language preferences and literacy levels.
- Navigate separate but shared caregiving (e.g., shared custody) and other types of living and caregiving arrangements.
- Consider how socioeconomic factors affect accessibility of your technology.
- Understand how caregiver roles change over time, including what information can be accessed, by who, when, and for how long.
Case studies


Key insights
Caregivers informing product development
This article explores the challenges and facilitators of parents using mobile health technology to monitor symptoms in infants with congenital heart disease. It identifies key factors that influence adherence to these monitoring practices, such as the usability of the technology, parental education, and emotional support. The analysis underscores the importance of developing user-friendly health apps that consider parents’ needs and concerns to improve adherence and outcomes for these vulnerable infants.
Insights from healthcare professionals:
Clinicians may resist new tools if they do not clearly see the value they provide to patients and families, or if the tools add complexity to their workflow. For DHTs to be effective, clinicians must trust the tools and be confident in their ability to improve patient outcomes. This trust can be significantly enhanced by embedding clinical subject matter experts (SMEs) in the development process to ensure that the tools are designed with clinical insight and relevance.
Key considerations from The Playbook team
Demonstrate value
Identify and quantify the needs and expectations of your clinical partners early on. Implement pilot studies to demonstrate how your DHT achieves these, and iteratively improve your product.
Test and iterate
Establish a continuous feedback loop with clinicians to refine and improve the tool based on their experiences and needs (e.g., within pilot studies).
Validate your solution
Clearly demonstrate the efficacy of your solution in real-world clinical settings on meaningful clinical and patient-centered outcomes.
Clinical champions
Find an early adopter and willing advocate of your DHT to educate their peers and drive adoption. Providers are more likely to adopt a solution if they have been taught by an “equal” person.
Clinicians play a key role in the successful adoption of pediatric DHTs. However, they often face heavy workloads and concerns over clinical validation and patient safety, limiting their adoption of new digital tools. Moreover, DHTs often add to, rather than decrease, the administrative burden healthcare professionals face, leading to fragmentation and low adoption. Finally, when DHTs do not have viable revenue streams (e.g., payment models), it is near impossible to drive adoption.
Key considerations from The Playbook team
EHR Integration
Design DHTs that integrate seamlessly with existing electronic health record (EHR) systems to streamline data sharing and clinical workflows. Learn more about the HL7 Fast Healthcare Interoperability Resources (FHIR) interoperability standards.
Clinician adoption
Tools must be intuitive and simple to integrate into workflows. Provide clinicians with proper training and resources to understand how to deploy the DHT, where appropriate, but focus on simplicity and integration first and foremost.
Reimbursement
Understand which reimbursement codes are applicable to your DHT (if any) and clearly demonstrate the revenue stream to your clinical partner. Without a viable billing and reimbursement pathway, the product is unlikely to be adopted.

Key insights
Exploring health care professionals’ perspectives on DHTs in pediatric care and rehabilitation
This article investigates healthcare professionals’ views on integrating DHTs into pediatric care. It highlights the perceived benefits, such as enhanced communication, improved patient monitoring, and increased access to care. However, the study also reveals concerns regarding the usability of these technologies, data privacy, and the need for appropriate training and support for both providers and families. Overall, it emphasizes the importance of engaging healthcare professionals in the development and implementation of DHTs to ensure they meet the needs of pediatric patients and their families.
Case studies





Key takeaways
User-centricity pays off
A user-centered approach isn’t just a strategy – it’s an investment that leads to better health outcomes, increased adoption, and long-term cost savings.
-

Increased engagement and effectiveness: Products developed with direct input from users are more likely to address real-world challenges and result in patient-centered outcomes. Children and caregivers are more likely to adopt and consistently use tools that are tailored to their needs and routines.
-

Reaching underserved populations: By incorporating insights from various demographic and socio-economic backgrounds, the holistic, human-centered approach promotes the development of equitable solutions that are accessible and effective for all populations.
-

Reduced development costs: Catching design flaws or usability issues early in development reduces the cost of rework and late-stage modifications. UCD leads to fewer iterations post-launch, reducing time-to-market and lowering overall development costs.
-

Increased clinical adoption: Products designed with clinician input are more likely to integrate smoothly into existing workflows and meet provider needs, making them easier to adopt and scale across health systems. Learn more about driving clinical adoption.












