Chapter 4 – Section 3
Measurement

Measuring changes in children can be exceptionally difficult given the variability in their development. Use this section to learn how DHTs enable reliable, robust, and consistent measurements in children and deliver more meaningful data.
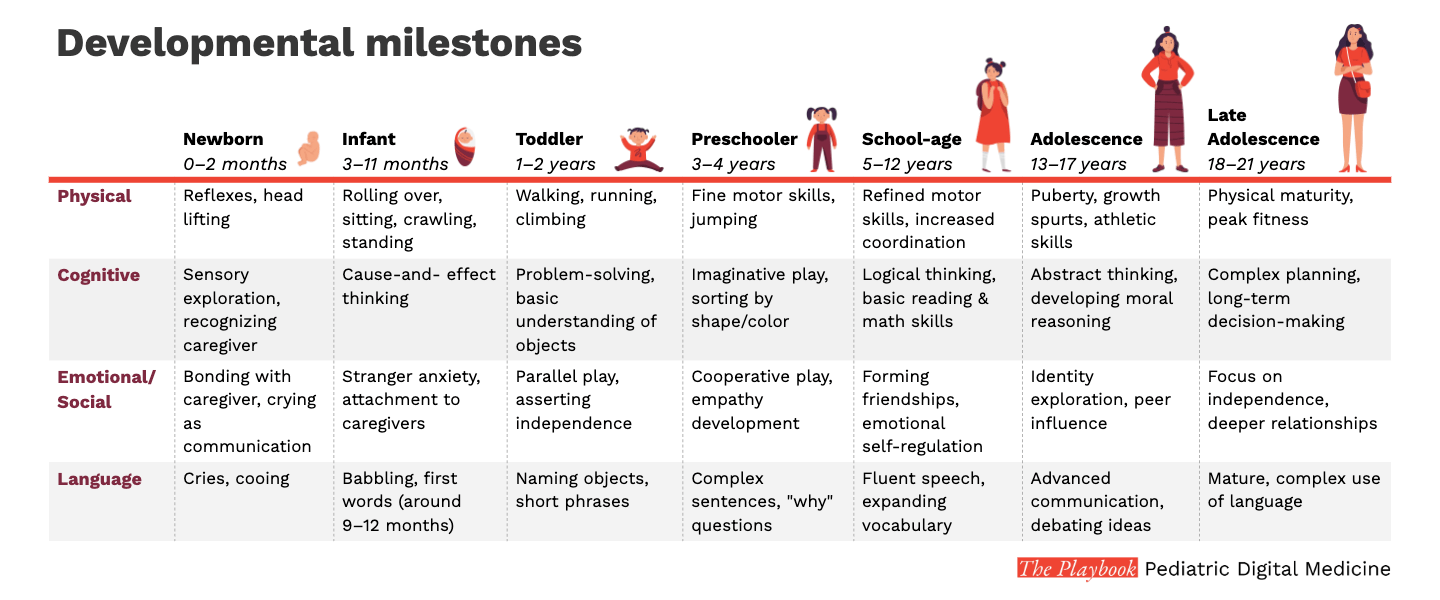
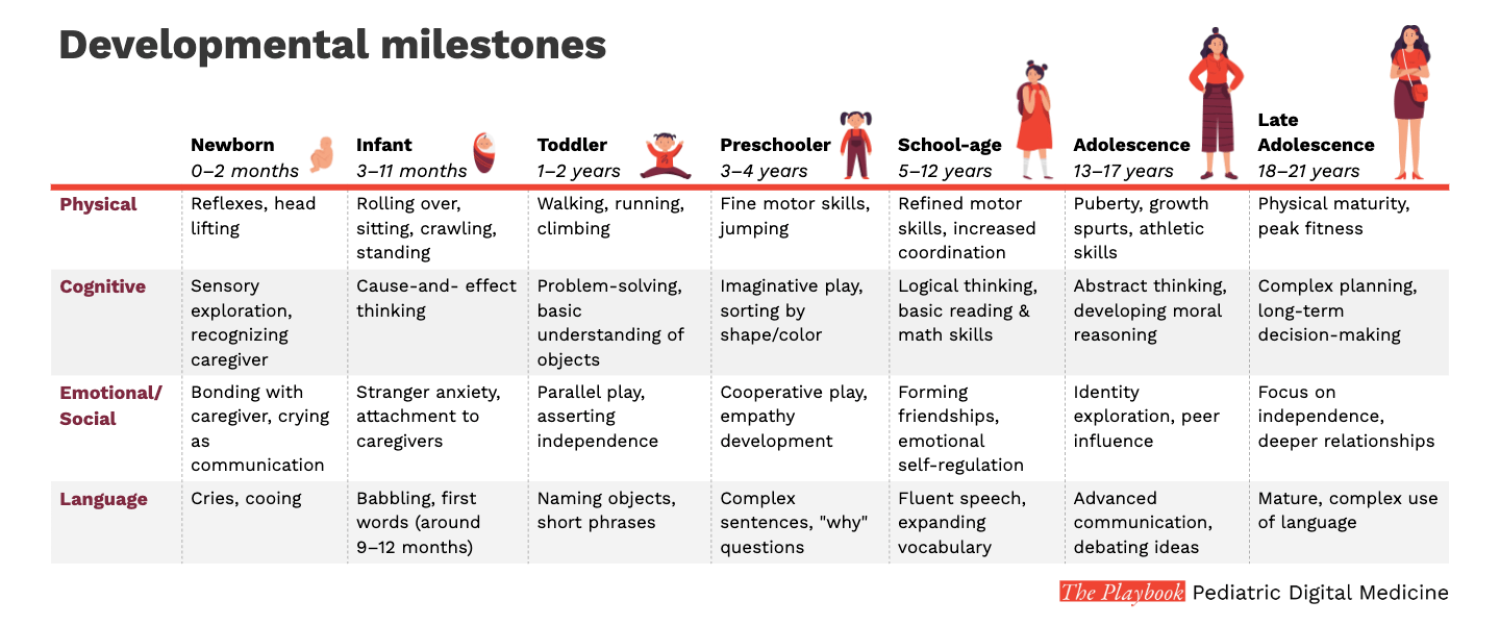
Measurement in children is difficult due to the pace and variability of their development
As presented in our earlier chapter, children age and develop in variable ways. These changes make obtaining accurate and meaningful measurements exceptionally difficult.

Meet Parker
The SV95c digital endpoint, a wearable sensor system, allows for more accurate, continuous measurement of upper limb function in children like Parker with DMD. By capturing real-world data on movement, this tool provides a more sensitive measure of subtle changes in his condition, offering the potential for better-informed clinical trials and ultimately leading to more effective treatments for DMD.
Personas illustrate key points in action, showcasing real-world applications and practical scenarios based on fictional personas.
Measurement tools and their challenges in pediatric research
There are various approaches to measurement in clinical trials. These clinical outcome assessments (COAs) include:
Patient-reported outcomes (PROs)
Self-assessment of how a person feels or functions in daily life, reported without modification of interpretation.
Observer-reported outcomes (ObsROs)
A measurement based on a report by a non-expert third party of how a person feels or functions in daily life. ObsROs are particularly useful for patients who cannot report for themselves (e.g., infants or individuals who are cognitively impaired).
Clinician-reported outcomes (ClinROs)
A measurement based on a report that comes from a trained healthcare professional after observation of a patient’s health condition.
A measurement based on standardized tasks performed by a patient that are administered and evaluated by an appropriately trained individual or are independently completed.
- Subjectivity: Many COAs, such as patient-reported outcomes, rely on subjective input, which can be challenging for young children who may not fully understand or articulate their symptoms.
- Snapshot data: Traditional assessments are often collected at discrete points in time, missing real-time fluctuations in a child’s health status and not adequately accounting for their rapid developmental changes.
- Limited engagement: Pediatric patients may struggle with engagement, leading to inconsistent data or compliance issues.
Clinical Outcomes Assessment (COA):
Assessment of a clinical outcome can be made through report by a clinician, a patient, a non-clinician observer or through a performance-based assessment. There are four types:
- Patient-reported outcome (PRO),
- Clinician-reported outcome (ClinRO) measures,
- Observer-reported outcome (ObsRO), and
- Performance outcome (PerfO).
Patient-Reported Outcome (PRO):
Self-assessment of how a person feels or functions in daily life, reported without modification of interpretation. Can be measured by self-report or by interview. Unobservable symptoms known only to the patient (e.g., pain severity or nausea) can only be measured by PRO measures.
Observer-reported outcome (ObsRO):
A measurement based on a report by a non-expert third party of how a person feels or functions in daily life.
A measurement based on a report of observable signs, events or behaviors related to a patient’s health condition by someone other than that patient or a health professional. Generally a parent, caregiver, or someone who observes the patient in daily life. They are particularly useful for patients who cannot report for themselves (e.g., infants or individuals who are cognitively impaired). It does not include medical judgement or interpretation.
Clinician-reported outcomes (ClinRO):
A measurement based on a report that comes from a trained healthcare professional after observation of a patient’s health condition. Most ClinRO measures involve a clinical judgment or interpretation of the observable signs, behaviors, or other manifestations related to a disease or condition. ClinRO measures cannot directly assess symptoms that are known only to the patient (e.g., pain intensity).
Performance outcome (PerfO):
A measurement based on standardized task(s) performed by a patient that is administered and evaluated by an appropriately trained individual or is independently completed. They require patient cooperation and motivation and include measures such as gait speed, memory recall or other cognitive testing.
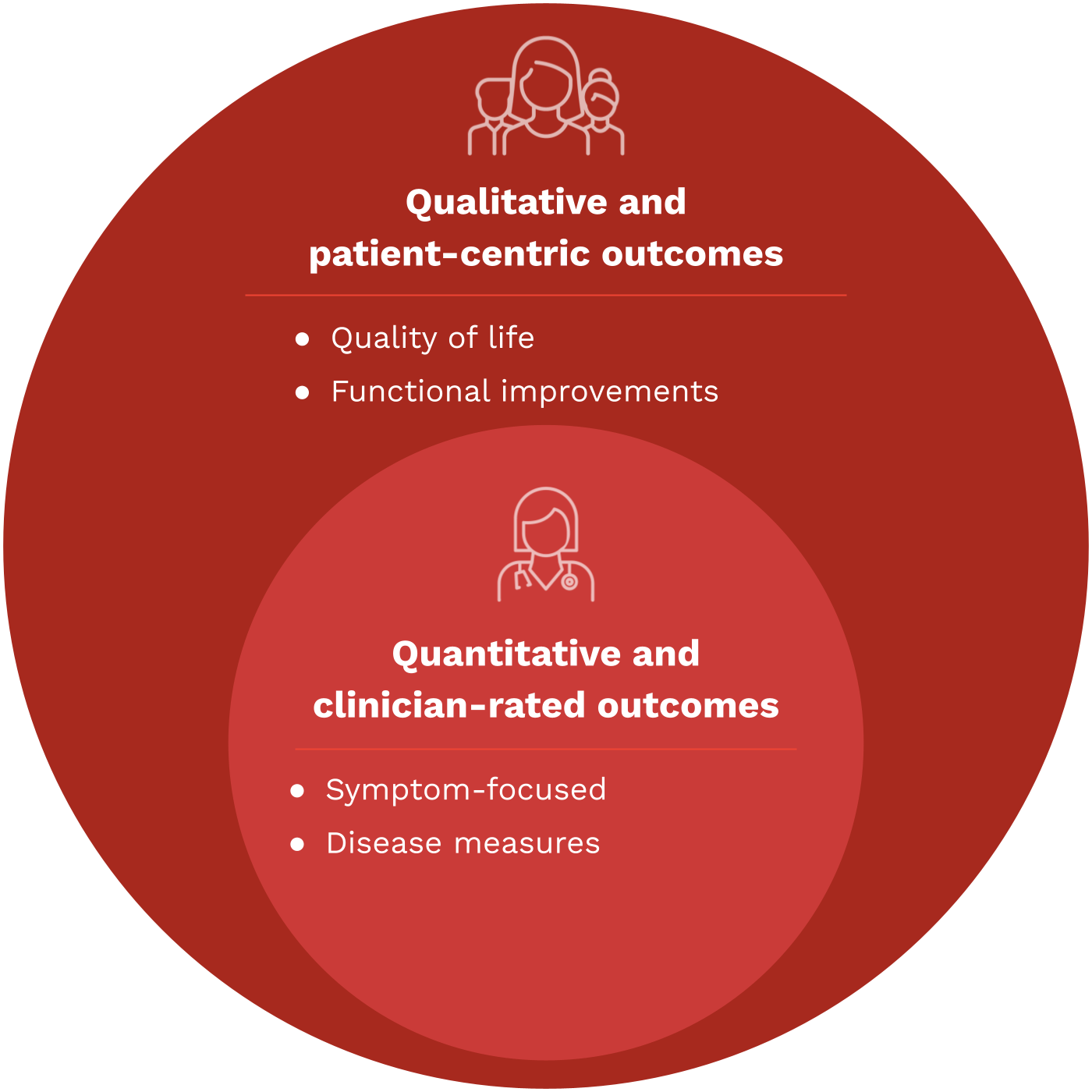
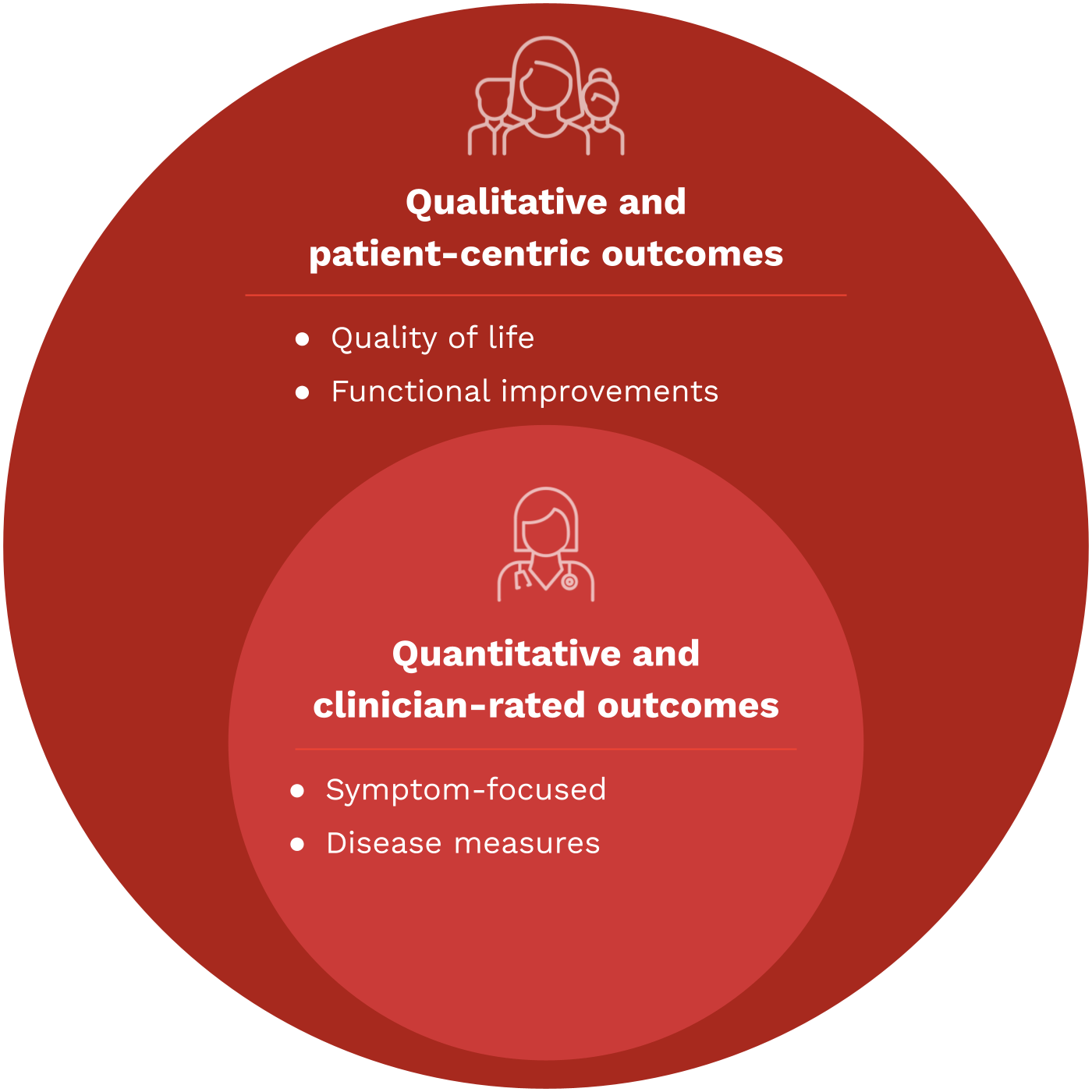
Beyond COAs: What is a meaningful measure?
Meaningful measures of health are specific metrics and endpoints that accurately reflect the health status, developmental progress, and overall well-being of children. These measures go beyond traditional clinical outcomes and are designed to capture what is genuinely important to patients and their caregivers.
Meaningful measures should be patient-centered, age-appropriate, relevant to pediatric populations, and transferable into relevant real-world outcomes and measures of improvement for actual patient care.
Patient centricity:
An approach that prioritizes the needs, preferences and values of patients, engaging them as active partners and decision makers in their own care. In the context of clinical trials, centralizing the patient (or participant) experience helps to:
- Guide the selection of meaningful outcomes,
- Reduce burden on patients and caregivers by improving trial efficiency,
Ensure appropriate privacy and data rights are protected throughout all stages of their development.
Bridging COAs, meaningfulness, and DHTs
DHTs offer innovative, real-time, and patient-centric solutions to overcome the challenges to traditional COAs, bridging the gap by:
- Enhancing data precision through continuous, objective monitoring.
- Capturing real-world patient behaviors, offering richer context.
- Adapting assessments to pediatric needs, improving engagement and accuracy.

Key insights
A patient-centred conceptual model of nocturnal scratch and its impact in atopic dermatitis
This study emphasizes a patient-centered approach to developing meaningful health measures for atopic dermatitis (AD), focusing on nocturnal scratching. By engaging patients and caregivers, the research identifies nocturnal scratching as a key symptom impacting health, and suggests that DHTs could provide objective, patient-relevant outcome assessments.
- Patient and caregiver insights underscore the effect of nocturnal scratching on sleep loss and quality of life in children with AD.
- Reducing night-time scratching is seen as a critical treatment goal by both pediatric patients and caregivers, highlighting its meaningfulness in managing AD.
- Using DHTs to measure nocturnal scratching aligns with patient priorities, offering an objective, relevant way to track treatment outcomes and disease progression in children.
Case studies

Digital endpoints can enhance objectivity and reliability
Digital collection of a measure does not make that measure inherently more reliable or accurate.
Digital endpoints offer fully objective measures of health outcomes collected using technologies such as sensors, wearables, and mobile applications. These measures transform continuous real-time data into precise and detailed insights of a patient’s health status or response to treatment.
Digital endpoints are the gold standard of measurement because:
- They must demonstrate their ability to accurately reflect changes in a patient’s condition, proving their validity and relevance to clinical practice.
- They meet the rigorous standards necessary for clinical decision-making and regulatory approval.
- Unlike traditional measures, digital endpoints offer objective, quantifiable insights collected in real time, which are crucial in pediatric research.
Digital Endpoint:
A precisely defined variable intended to reflect an outcome of interest that is 1) collected digitally with a sensor, and 2) statistically analyzed to address a particular research question.

Key insights
Playbook team analysis – Developing a reliable digital endpoint and enhancing data collection

Challenge
- Digital endpoints are used far less frequently in clinical trials for pediatric populations than in clinical trials for adult populations. This disparity is because there are few DHTs validated and cleared for use in pediatrics.

The Approach
- The technology: Activity level and vital signs are disease-agnostic measures of general health. Wrist-worn watches and patch-based devices use multiple sensors to capture and analyze both activity level and common vital signs, such as temperature and heart rate, concurrently. The sensors provide continuous, quantitative data on these measures of health in real-world settings, allowing for data collection outside of the clinic.
- Validation studies: A study focused on validating the accuracy of the wearable sensors for activity level and vital signs in pediatric populations under various activity conditions was conducted. Participants also completed a decentralized study component aimed at assessing usability and acceptability by wearing at home for 24 hours.
- Solution: Three DHTs were validated for accuracy and assessed for at-home usability in children ages 2-12.

Result
- It is essential to evaluate DHTs for use in pediatric populations prior to their use as digital endpoints. Wearable sensors must be compatible with the physical size, cognitive level, and lifestyle of the child, and the support of caregivers in using DHTs at home should be considered.
Digitally enabled measurement in pediatric trials

Validation:
- Measures should account for the rapid and varied developmental changes (physical, cognitive, emotional, and social) that occur in children.
- Assess the quality, performance, and clinical relevance of the outcome measure generated by these tools. (Reference DiMe’s V3+ framework as the de facto standard across the industry.)
- Adhere to consistent data formatting across platforms for better comparability and wide-scale impact.
Relevance and implementation:
- Integrate digital endpoints with traditional clinical measures and patient-centered outcomes to ensure that healthcare providers can use and interpret data in a meaningful way.
- In order for true translation, measures should be interoperable with EHR systems and incorporated into clinical decision support systems (understanding, willingness to engage) without introducing bias.
- Ensure measures can be reliably and consistently collected across different settings and populations.
- Consider the burden of data collection on patients and families, aiming for methods that are non-invasive and feasible. (Read more about data collection in pediatrics here.)