Just over a year ago, we launched our crowdsourced library of digital endpoints, aiming to shine a light on digital measures being used in industry-sponsored trials and galvanize the field around specific measures to speed adoption. During our most recent update of the library, we were struck by the astronomical growth of digital endpoints over such a short time span.
Let the numbers speak for themselves:
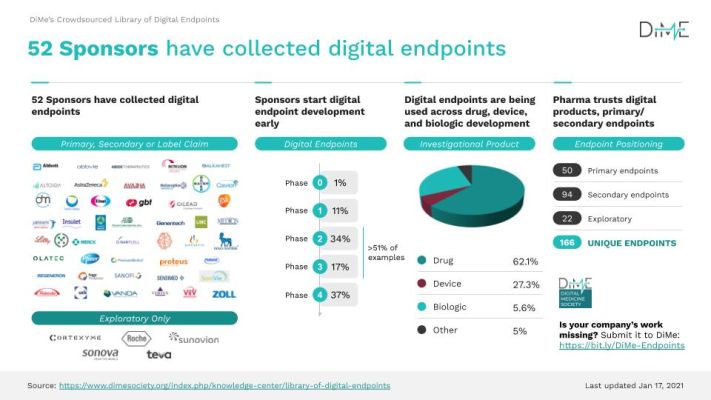
- The number of unique digital endpoints increased from 34 to 166 in the last 14 months, and the number of sponsors actively collecting digital endpoints in clinical trials of their medical products has increased from 12 to 52.
- Last year, 50% of digital endpoints being collected were being used in drug trials — this has increased to 62%, with relatively fewer being used in device and biologic trials.
- There has been an increase in the use of digital clinical endpoints during the post-market phase of drug and biologic trials and dwindling in early phase trials, whereas their use in Phase 2 and Phase 3 have held quite steady.
- Across investigational products, the positioning of digital endpoints has also changed dramatically: at the library’s initial launch, roughly 40% of digital endpoints were exploratory; now, they only account for just over 10% — indicating increasing confidence in their use.
Search DiMe’s crowdsourced library of digital endpoints at https://dimesociety.org/knowledge-center/library-of-digital-endpoints
Instinctively, this growth in the number of digital endpoints being used in medical product development — and the number of sponsors who have embraced them — feels like a good thing. But is more always better?
Determining “Real” Growth
No doubt, the pressure of the COVID-19 pandemic catalyzed an embrace of digital clinical measures and other decentralized approaches to clinical research that few of us could have imagined a year ago. However, despite a five-fold uptick in the use of these measures, we’ve yet to see a new medical product approved on the basis of a digital efficacy endpoint.
There is one digital endpoint that was qualified by the European Medicines Agency (EMA), Stride Velocity 95th centile as a Secondary Endpoint in Duchenne Muscular Dystrophy Measured by a Valid and Suitable Wearable Device. Of note, this was the result of significant and sustained pre-competitive collaboration.
In the U.S., seven digital clinical outcome assessments (COAs) have been submitted to FDA’s COA Qualification Program. Yet, no digital biomarkers have been submitted to FDA’s biomarker qualification program. This is desperately disappointing as “digital biomarkers” is one of the biggest buzzwords of the pandemic.
And the same trend appears in DiMe’s endpoints library: to date, we’ve not seen a single digital endpoint in the library appear more than once. Of all 166 digital endpoints listed, Every. Single. One. Is. Different.
The other day, someone very kindly congratulated our team on the library, reflected on its growth, and said, “Imagine… in another year there could be 1,000 digital endpoints in the library! Wouldn’t that be great?”
There aren’t 1,000 meaningful things to measure! And, until we collectively work to identify the best concepts of health to measure digitally for a given patient population, all of this upstream work will yield limited progress. We must start honing in on common digital approaches to measuring a given health concept. If we don’t, it will be an uphill battle to establish normative data sets and understand the natural history of disease, as described by digital clinical measures.
What’s more, we must also focus on the best digital clinical measures — and, in turn, the best digital endpoints. The last thing we need is a proliferation of digital measures of the same ilk as the six-minute walk test: a poor quality, inefficient, and widely overused measure of convenience.
Where Do We Go from Here?
DiMe’s digital endpoints library is a powerful resource, benchmarking progress in the field and highlighting the work we must continue to do to advance the use of digital endpoints to speed medical product development.
Anecdotally, I know of at least nine industry sponsored trials that included digital endpoints because the groundswell of activity documented by our library added confidence to the decision to move ahead. The work of our community in developing and maintaining this crowdsourced resource is critical to the field.
So then, how do we continue to expand adoption? What is the next step toward establishing a shared menu of high quality, meaningful digital endpoints? Bemoaning the fact that harmonization in the selection and development of digital endpoints isn’t quite happening yet is about as useful as a coach standing at the side of the track yelling at their athletes to “run faster!”
Well, yes. Obviously… but, how?
Enter The Playbook
Seeking harmonization of high-quality digital endpoints at the last step of the process is not a viable strategy. It is critical that shared best practices for the whole process — from digital clinical measure selection, through development and evaluation, all the way through to deployment — are established and broadly adopted.
In September 2020, we launched The Digital Clinical Measures Playbook (The Playbook) along with multi-stakeholder colleagues from Elektra Labs, Genentech, Koneksa, Myokardia, Sage Bionetworks, Scripps Research, and the US Food and Drug Administration (FDA). This resource synthesizes best practices from the digital health field, breaking down silos to create one comprehensive and accessible “how-to” guide to support all stakeholders working to advance the safe, effective, ethical, and equitable use of digital clinical measures to improve lives.

Our goal in developing The Playbook was to solve the “first mile problem’” and give the field a shared foundation for how to select and develop digital clinical measures and guide for successful deployment. The Playbook outlines a vision for harmonization, covering the use of digital clinical measures in healthcare, as well as in public health decision making and policy.
A mere six weeks after launch of The Playbook, we kicked off a Tour of Duty, “The Playbook: Driving Adoption”, to solve the “last mile problem”: establish The Playbook as the gold-standard for developing and deploying digital clinical measures. This Tour is well underway, with plans to launch a suite of resources to support the broad adoption of core elements of The Playbook on April 30.
A Rise in Collaboration
Perhaps the most impressive characteristic of this Tour is the breadth of pre-competitive collaboration. Amid the greatest public health crisis of our lifetimes, companies from life sciences, healthcare, and technology have come together alongside patient experts and regulators to drive adoption of The Playbook as the foundation for digital measurement.

Collaboration around measures is in the best interest of the patient: it accelerates broad trust, understanding, and acceptance of digital clinical measures across use cases.
Yet pre-competitive collaboration in digital clinical measure development is rare, with a few notable exceptions such as the industry collaborative pursuing FDA qualification of an activity monitor-based endpoint measuring physical activity led by the C-Path’s PRO Consortium, the Critical Path for Parkinson’s 3DT initiative, and IMI funded activities such as MOBILISE-D, RADAR-CNS, RADAR-BASE, and IDEA-FAST.
Our DiMe team is playing a significant role in making this rarity more mainstream, hosting the leaders in our field committed to pre-competitive collaboration to advance the field. We are the only group solely focused on establishing methodological best practices in digital measurement across the full spectrum of healthcare. Together, we are committed to developing the blueprint for developing and deploying digital clinical measures that will establish truly gold standards in the digital era of health.
How You Can Help
At DiMe, we’re doing what we’re best at: finding the most pressing problems in the field of digital medicine and attacking them with solutions from every angle. And there’s more to be done. Help us continue to push for more transparency, clarity, and solutions to progress our field:
- Partner with us to keep DiMe’s digital endpoints library up to date by submitting your digital endpoints here.
- Engage in the comment process, sign up for updates, and access educational resources to help us further establish The Playbook as the gold standard for digital clinical measure development.