
The time is now: Enabling health equity with digital tools and partnerships
The healthcare ecosystem, including clinical trials, is experiencing many pain points –workflow inefficiencies, financial burdens, and worsening health outcomes, to name a few. One very big problem is that many populations continue to be underserved or underrepresented in healthcare. This is a health equity problem. Or rather, the lack of health equity problem, which has manifested from decades of systemic inequities and injustices.
The COVID-19 pandemic spotlighted consequences of the lack of health equity. Systemic racism has also produced a clinical trial system, where either the majority of participants are White or information on race and ethnicity are rarely collected. The clinical trials system has a diversity problem, an equity problem, and an inclusion problem. Each of these entities are important separately, and together, and all need to be addressed to advance health equity.
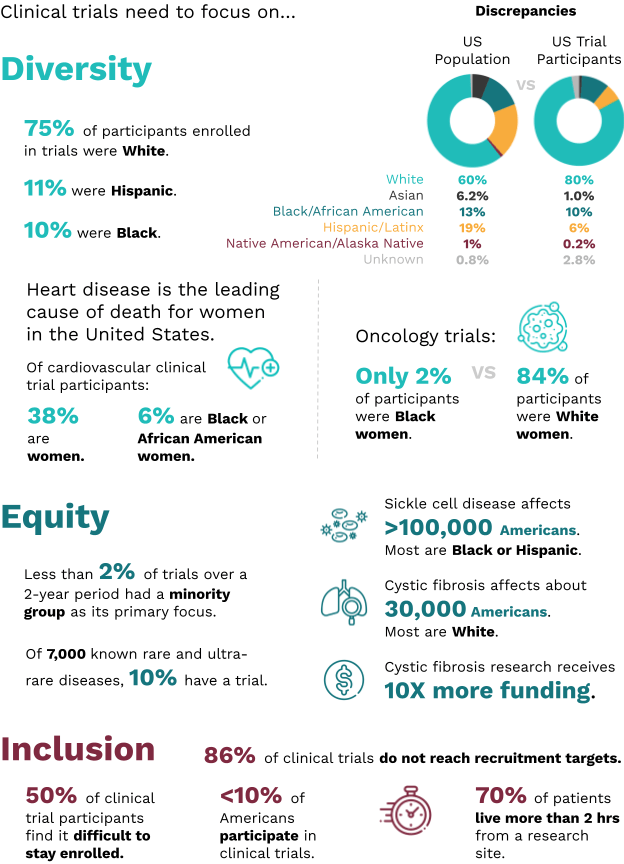
See full image here
The digital revolution is promising to mitigate many of these pain points and to transform healthcare and clinical trials. Transforming healthcare through the use of digital technologies is central to work conducted by the Digital Medicine Society (DiMe). And, with a program delivery model fueled by interdisciplinary collaborations, DiMe was poised to take on a multi-dimensional project such as health equity. A little less than a year ago, DiMe convened and challenged stakeholders from across the clinical trials ecosystem to identify opportunities to increase and improve diversity, equity, and inclusion in digitized clinical trials. With the increasing innovations and use of digital tools in clinical trials, the time is right to ensure that diversity, equity, and inclusion are prioritized.
New resources to drive DEI in digitized clinical trials
DiMe’s Diversity, Equity, and Inclusion in Digitized Clinical Trials project team represents patients, patient advocates and engagement specialists, clinical trial sponsors, decentralized clinical trial organizations, digital solution innovators, life sciences, and researchers. By exchanging real-world experiences with previous diversification efforts, sharing experiences with the lack of diversity and equity in clinical trials, and ideating on current real-world challenges, it became clear that cross-industry partnerships can enable the use of digital tools to advance diversity, equity, and inclusion in clinical trials, today. The project team then created resources that will allow those designing and implementing clinical trials to take specific actions to advance health equity. The resources are organized around three steps: assess, identify, and implement.
- Assessing the opportunities for applying digital tools for a more diverse, equitable, and inclusive trial begins with understanding the need for health equity and applying digital tools to address many of the challenges facing traditional clinical trials. This is the digitization of clinical trials, that is using digital tools to uplevel traditional processes from participant engagement and enrollment to protocol design and data collection. Building on the elements of a digitized clinical trial publication from FDA Commissioner Robert Califf and other experts, the project team emphasized the need to start with an assessment of digital readiness.
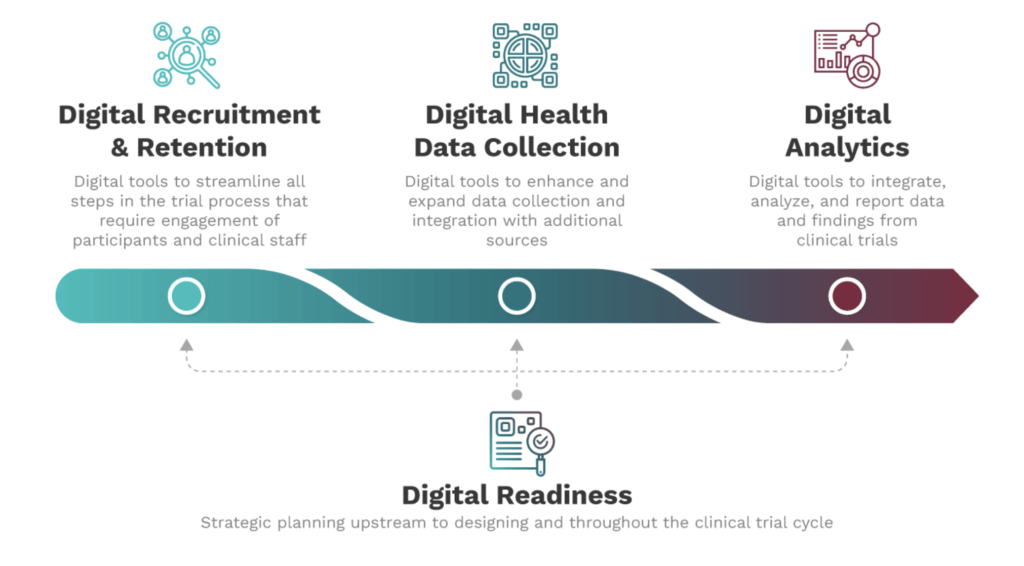
See full image here
- During identification, you can use the “Elements of a Digitized Clinical Trial” to learn how each digital tool can be used in each step of the digitized clinical trial. Project team members Alteha.ai and Rubix LS use digital tools like Artificial Intelligence/Machine Learning (AI/ML) and Real-World Data and Real-World Evidence (RWD/RWE) to identify diverse populations for engagement and enrollment. The tools of digital marketing and digital platforms are used by project team members Reveles, Amgen, GSK, and Inlightened to build trust through community partnerships and increasing representation of clinical trial teams. Project team members Medable and Sage Bionetworks use eConsent to engage and inform participants through more user-friendly mechanisms, while increasing efficiencies for clinical trial teams.
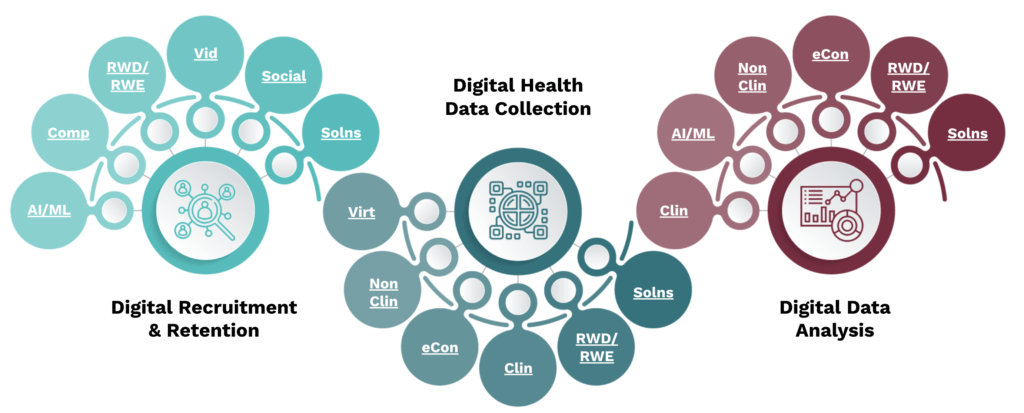
See full image here
- The implementation step provides resources around specific actions that can be incorporated into a diversity plan, per the FDA draft guidance. These specific actions are focused on developing a person-centered strategy around diversity, inclusivity, and accountability. Through community engagement and partnerships and participant empowerment clinical trial designers and implementers can begin to improve the enrollment of participants from underrepresented groups. The accountability piece calls for everyone in the clinical trial ecosystem to hold themselves and others in the industry accountable for developing and implementing these specific actions.
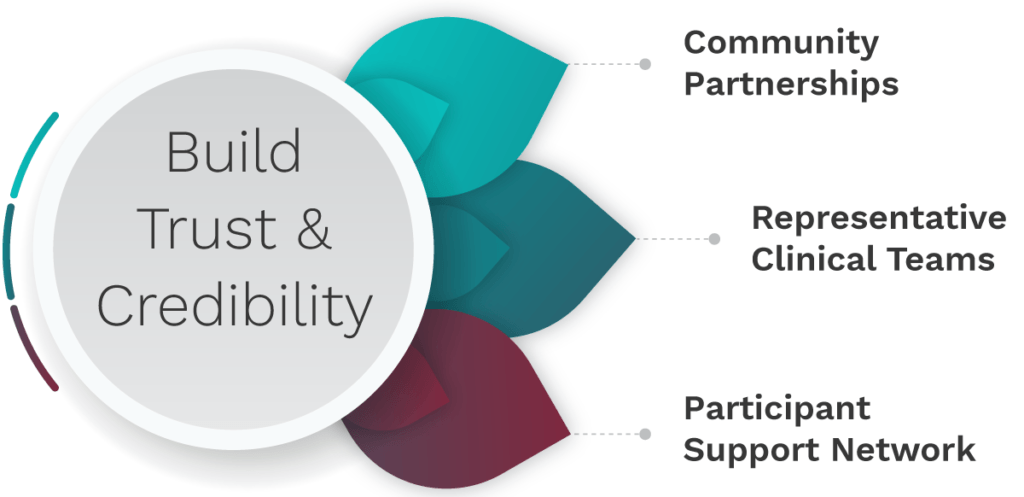
See full image here
Watch the full launch of these resources.
The power of partnership
Creating these resources with a cross-disciplinary project team emphasized the value of partnerships and cross-collaborations. These industry leaders have been working on clinical trials and diversity for many years, and have come to appreciate the value that partnerships can bring to their efforts. Clinical trial sponsors, decentralized clinical trials and clinical research organizations, or even clinical research sites cannot fix the diversity, equity, and inclusion issues alone. Working together with patient advocates and engagement specialists allows these entities to address multiple challenges at once, including access to diverse participants and innovations for developing more equitable and inclusive trials.
Several project team members are already harnessing the power of partnerships. Genentech partnered with TOUCH, the Black Breast Cancer Alliance to support and promote the representation of black women in cancer trials. More recently, Lightship and Acclinate partnered to increase access and engagement of diverse participants for a neurology trial. These types of partnerships will allow the different clinical trial stakeholders to learn from each other’s past efforts and address many more challenges simultaneously. All of these efforts can be enhanced with the resources created by the project team.
The journey ahead
Advancing health equity in clinical trials can be a long process, however, the resources launched by this project team will help to speed the process while allowing the overall healthcare system to be transformed by digital tools. It would be short-sighted to promote the use of digital tools for advancing diversity, equity, and inclusion without mention of the digital divide. The digital divide is a big reality for many underserved and underrepresented populations. However, digital is transforming every aspect of our lives, and consumers are learning to embrace these technologies. Healthcare, including clinical trials, is evolving with digital tools. It is imperative that all stakeholders in the healthcare and clinical trial ecosystem work together to ensure that all populations have what they need to fully participate. The health equity in digital medicine work will continue through the Digital Health Measurement Collaborative Community (DATAcc) initiative. Sign up for the DATAcc newsletter to get updates on this and other digital health measurement work. In the meantime, the resources created by the Diversity, Equity, and Inclusion in Digitized Clinical Trials project show how you can get started. We invite you to use these resources and share with us how you are moving beyond talking about this problem and working to transform the system.



