Imagine you’re an entrepreneur with a groundbreaking digital health product that’s changing lives in one country. You’re ready to expand globally, but the regulatory maze in each market is a puzzle. Where do you start?
This is the problem DiMe’s International Regulatory Pathways project team set out to solve. The team built a set of resources to support digital health technology (DHT) innovators with global regulatory insights to support their go-to-market strategies in environments across Asia Pacific, Europe, and North America.
To understand how these resources are shaping the future of digital health, we spoke with Dr. Ariel Dora Stern, an expert at the intersection of innovation, regulation, and strategy in healthcare. As the Alexander von Humboldt Professor for Digital Health, Economics and Policy at the Hasso Plattner Institute and a Full Professor at the University of Potsdam, Dr. Stern brings a wealth of experience from her research on how medical technologies are adopted and the policies that influence them along with several years of advising digital medicine startups.
How it began
Dr. Stern’s relationship with DiMe began in 2019, during her time organizing the inaugural Harvard Digital Medicine Symposium. She recalls being urged to meet Jennifer Goldsack, who was launching DiMe, an organization focused on evidence-based digital medicine.
“Jennifer joined us at the symposium, and DiMe officially launched at that event,” Dr. Stern shared. “It was thrilling to see this bold vision take shape at a moment I had the privilege to host.”
From that day forward, Dr. Stern has remained a contributing partner and advocate to DiMe’s mission of advancing digital health through collaboration and innovative tools.
Innovating globally
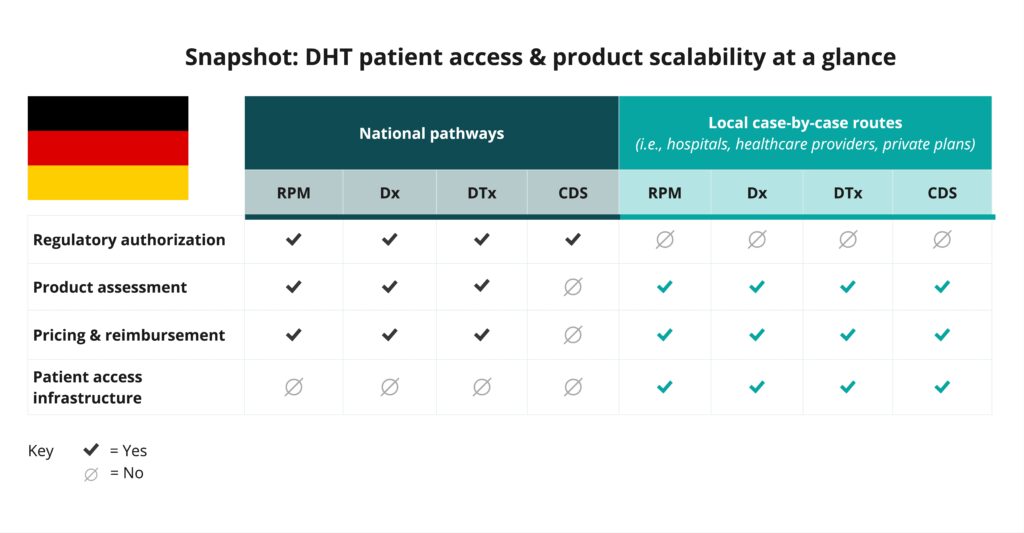
Dr. Stern’s academic work often focuses on how regulations and firm strategy intersect, particularly in the adoption of 21st-century healthcare technologies like digital health products and precision medicine. During her sabbatical with Germany’s Health Innovation Hub, an independent think tank associated with the German Ministry of Health, she witnessed the launch of the Digital Health Applications (DiGA) program, which set the stage for regulating and reimbursing digital health applications.
“Healthcare is not a country-specific problem,” Dr. Stern explained. “When we create transformative innovations, we create them for patients everywhere. But the regulatory frameworks that guide these innovations remain fragmented and difficult to navigate.”
Dr. Stern points to DiMe’s regulatory tools as a critical solution to this problem. From the U.S. pathways project to the recently launched International Regulatory Pathways project, these resources clarify where innovators need it most.
Her experience has taught her that innovators everywhere face similar questions. “Whether it’s Germany or the U.S., startups are asking the same things: How do we enter this market? What’s required? DiMe’s tools bridge that knowledge gap.”
Dr. Stern sees the IRP resources as more than guides—they’re shaping the foundation of a young and rapidly evolving field.
“One of the biggest challenges in digital medicine is the lack of standardization,” Dr. Stern explained. “There’s so much variability in how research is conducted and presented. DiMe’s tools help establish a shared language, which benefits innovators, regulators, and ultimately patients.”
She described this as a “procyclical effect,” where standardized evidence-generation practices lead to faster, more predictable regulatory evaluations. This, in turn, builds momentum for further innovation and adoption.
The power of collaboration
For Dr. Stern, working on these projects with DiMe isn’t just about creating tools; it’s about the collaborative spirit that drives the organization.
“I’ve learned so much from being part of these diverse conversations,” she said. “Hearing directly from startups, academics, and policymakers opens up new perspectives. It’s a feedback loop of learning and growth that I’m thrilled to contribute to.”
Dr. Stern is excited about the IRP resources and their potential to transform digital medicine. She envisions startups using these tools to navigate global markets with confidence and clarity, backed by evidence-based strategies.
“Imagine telling an investor, ‘We’ve mapped out what it takes to enter these key markets, and our strategy aligns with DiMe’s published frameworks,’” Dr. Stern said. “That’s a powerful story to tell—and it’s one that inspires confidence.”