

France National Companion Guide
Pursue DHT market access in France
Feedback
These pathways are constantly evolving. Work with us to keep them up to date by providing suggestions and feedback.
In France, digital health technology (DHT) product regulatory authorization, value assessment, and pricing and reimbursement are conducted at the regional and national levels. However, developers often pursue patient access infrastructure via local routes.
Use the resources below to explore pathways for scalability and to start planning your regulatory strategy to bring your DHT to market in France.
What is a policy “full-stack”?
A policy “full-stack” provides a structured approach for integrating digital health technologies (DHT) into national healthcare systems.
Components of a comprehensive national policy for DHTs
Regulatory authorization
Product value assessment
Patient access infrastructure
Pricing & reimbursement
Read more about the core elements of a national policy “full-stack” approach.
This guide reflects current information as of 15 October 2024. National regulations and practices are subject to change and evolve, so developers are encouraged to regularly consult relevant agency websites for the latest information.
Explore DHT patient access and product scalability pathways
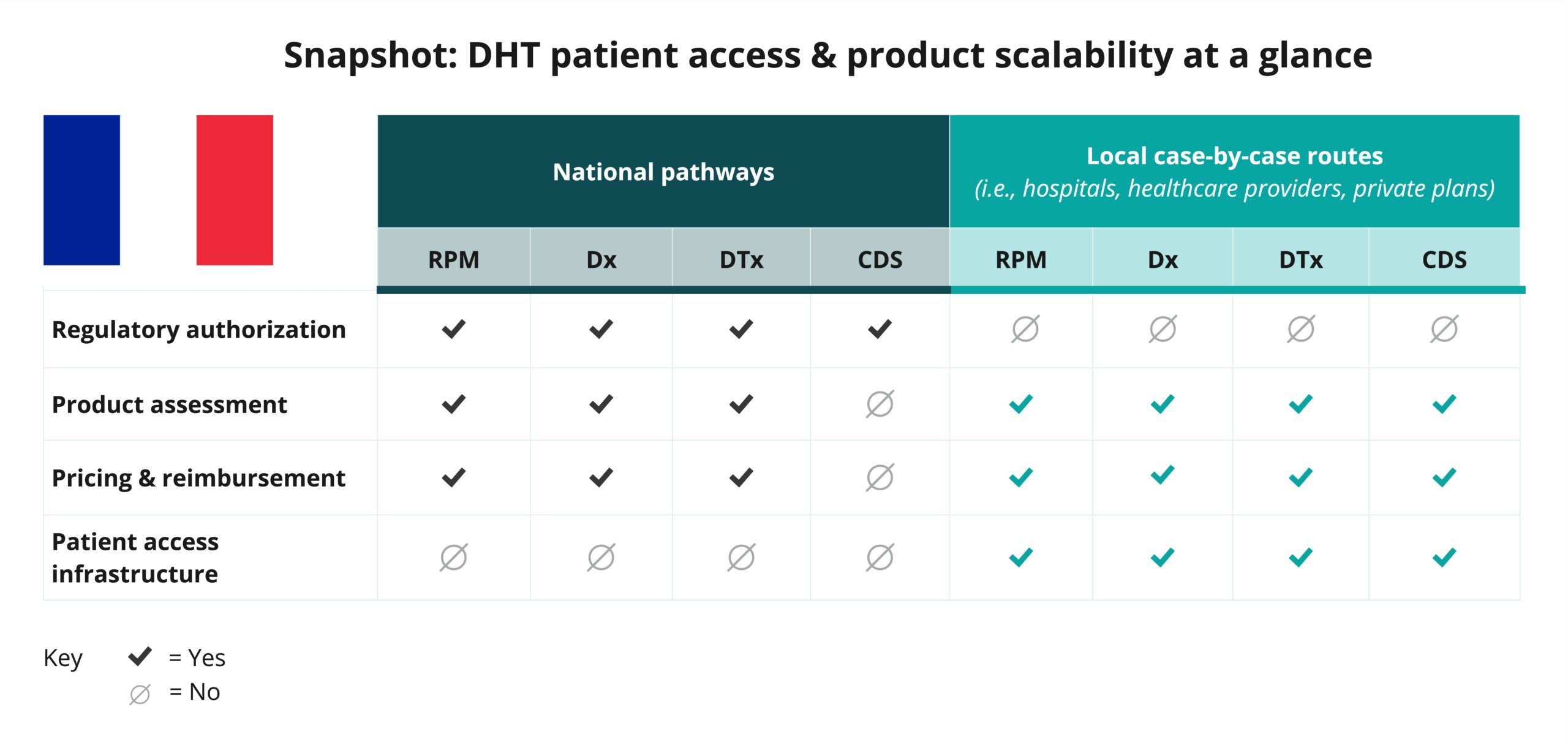
Begin your assessment of France’s routes to DHT patient access by reviewing this snapshot, which offers insights into the potential patient access and product scalability pathways available for remote patient monitoring (RPM), digital diagnostic (Dx), digital therapeutics (DTx), and clinical decision support (CDS) products.
Navigate national pathways for DHT patient access
And then, use this flowchart to help you navigate France’s national pathways for DHT market access, guiding you through key steps in regulatory review, product assessment, pricing, reimbursement, and patient access. Aligned with the four core elements of a national policy “full-stack,” it focuses on national pathways and does not provide detailed insights on local routes.
As you move through the flowchart, you’ll see where developers need to generate or present clinical evidence to demonstrate each product’s safety, efficacy, and impact. “Clinical evidence evaluation” refers to a review of published data, while “clinical investigation” highlights when you may need to conduct a study to verify your product’s safety and performance.
Use this flowchart to stay informed at each stage and understand what’s expected to bring DHTs to patients in France.
This flowchart:
- Provides a sample of important steps along the pathway to patient access—not including post-market assessment pathways—and is subject to change. Other exclusions may exist.
- References DHT products that qualify as a medical device. DHT products that are not recognized as medical devices are not represented in this flowchart.
- Focuses primarily on national pathways and does not provide detailed insights on local case-by-case routes.
- Identifies certain opportunities to generate clinical evidence evaluations and investigations but does not incorporate health economic outcomes research (HEOR).
- Does not include specific timelines and is not intended to indicate how long or when steps are conducted.
National policy “full-stack” components
As indicated in the snapshot, DHT product regulatory authorization, value assessment, and pricing and reimbursement in France are conducted at the regional and national levels.
Click below for more insights
- The marketing of medical devices in European Union (EU) member states is now governed by the European Union (EU) Medical Device Regulation (MDR) (Regulation 2017/745), which was first enacted on 26 May 2017 and became mandatory for medical devices in France beginning 26 May 2020.
- General medical device classification requirements for the EU are set out in Annex VIII of Regulation 2017/745. Per the regulation, “Software, which drives a device or influences the use of a device, shall fall within the same class as the device. If the software is independent of any other device, it shall be classified in its own right.”
- In France, products such as remote patient monitoring (RPM), digital diagnostics (Dx), and digital therapeutics (DTx) that qualify as medical devices may be classified into one of four categories: Class I, IIa, IIb, or III. Clinical decision support (CDS) products are not typically incorporated into national pathways.
- There are three possible funding pathways for digital medical devices (DMDs) – the most common way to refer to DHTs that qualify as a medical device – in France:
- Innovation funding: For innovative medical devices that are likely to have a clinical or medico-economic benefit.
- Prise en charge anticipée numérique (PECAN): For a DMD presumed to be innovative with possible ongoing studies to demonstrate the clinical or organizational benefit of the solution.
- PECAN is a one-year transitional and temporary reimbursement scheme for DMD therapeutic purposes (DTx) or telemonitoring products (RPM) that are in classes I, IIa IIb, and III. It was launched in April 2023 to accelerate patient access to useful innovations and provides:
- One year reimbursement with clear rates and a pathway to obtain long-term reimbursement.
- Access to a fast-track application, with a parallel evaluation of the application on the basis of initial evidence.
- Operational deployment in parallel with the procedures for permanent reimbursement.
- Between April 2023 to September 2024, there were 17 applications initiated for PECAN, with 2 successful applications, 5 failed applications, and 1 withdrawn application.
- PECAN is a one-year transitional and temporary reimbursement scheme for DMD therapeutic purposes (DTx) or telemonitoring products (RPM) that are in classes I, IIa IIb, and III. It was launched in April 2023 to accelerate patient access to useful innovations and provides:
- Common law via Liste des Produits et Prestations Remboursables (LPPR) and Liste des activités de télésurveillance médicale (LATM): For an individual-use medical device that has shown benefit.
Liste des Produits et Prestations Remboursables (LPPR)
Being listed on LPPR allows the product or service to be reimbursed directly by the Health Insurance Fund (otherwise known as standard coverage). The LPPR defines a reimbursable amount for each product. The price of a LPPR product may be higher than the amount reimbursed by the Health Insurance Fund. The difference is covered by the patient, their supplementary health insurance, or another organization.
The Haute Autorité de Santé (HAS) evaluates the clinical studies submitted by the manufacturer and “classifies” the medical device according to the expected service and the improvement of the expected service for the patient. The coverage rate is then set after negotiation with the Economic Committee for Health Products (CEPS). There is no predefined range to regulate these amounts.
Liste des activités de télésurveillance médicale (LATM)
This system allows access to the market for remote monitoring activities under France’s standard healthcare system and for such activities to qualify for reimbursement by the Health Insurance Fund. Two flat fees are paid per patient:
- One technical fee is paid to the company operating the telemonitoring DMD; and
- One medical fee is paid to the care team managing the patient.
A manufacturer cannot bill the technical fee without the corresponding medical fee billed by the care team. The monthly rates, which cannot be combined, are set for each patient as follows:
- For an organizational benefit: €50, all taxes included.
- For a clinical benefit relating to quality of life: €73.33, all taxes included.
- For a clinical benefit relating to morbidity: €82.50, all taxes included.
- For a clinical benefit relating to mortality: €91.67, all taxes included.
Prise en charge anticipée numérique (PECAN)
- The financial compensation for digital therapy for individual use (DTx) per patient:
- Initial fee (1st trimester): 435 € incl. VAT.
- Initial fee is followed by 38.3 € incl. VAT per month from 4th month.
- Total of 780 € incl. VAT max per year.
- Note: There is no remuneration for healthcare professionals.
- The financial compensation for remote medical monitoring solutions (RPM) per patient:
- Monthly technical fee: 50 € incl. VAT, adjusted based on the total average active telemonitored patients in the same indication (ICD-11, level 2) for PECAN or LATM.
- Note: Predefined remuneration is set for healthcare professionals.
- The French government is undertaking efforts to digitalise the healthcare system. While no national electronic prescribing system is in place, a recent law requires that all prescriptions should be digitized and linked to a national database.
- “Mon Espace Santé” provides patients with a central location to store and share with medical professionals information related to illnesses and health issues, treatments, allergies, and vaccinations.
- The G-NIUS platform, hosted by the Ministerial eHealth Delegation (DNS) and the Agence du Numérique en Santé (ANS), is a website provided by the government with advice and information for manufacturers, such as:
- Helping entrepreneurs identify the subjects that must be integrated into their tools (how to manage health data, etc.).
- Overview of all the main eHealth players in France.
- Navigating the reimbursement system to build a business model and identify the various types of public funding in France and Europe.
Curated overview of clinical evidence requirements and practices
To achieve a Conformité Européene (CE) mark via a notified body at the European level, medical devices must pass a conformity assessment, which certifies that the product meets legal requirements, is safe, and performs as intended. However, to demonstrate impact for reimbursement in France, developers must generate clinical evidence showing that the product performs better than the standard of care.
France provides DMD developers with numerous resources on clinical trial requirements and guidelines through websites such as Agence nationale de sécurité du médicament et des produits de santé (ANSM) and G_NIUS.
Click below for more insights
For the PECAN fast-track pathway in France, clinical evidence evaluations should be underway by the time of application to the program, particularly given the timelines needed to apply to either of the two common-law LPPR or LATM programs.
- For products applying through the PECAN program for inclusion in the list of reimbursed telemonitoring activities (LATM), developers need to apply for definitive reimbursement under ordinary law no later than 9 months into the 12-month PECAN period. This timing allows for a three month evaluation by the HAS National Committee for the Evaluation of Medical Devices and Health Technologies (CNEDiMTS) prior to a determination of permanent reimbursement.
- For products applying through the PECAN program for inclusion in the list of reimbursable products and services (LPPR), developers need to apply for definitive reimbursement under ordinary law no later than 6 months into the 12-month PECAN period. This allows for a 3-month evaluation by the CNEDiMTS and a 3-month price negotiation with the CEPS prior to a determination of permanent reimbursement.
- While HAS accepts studies conducted outside of France for the PECAN pathway if multiple conditions are met, public-facing examples of what this process looks like in practice are not available.
A more complete listing of resources may be found in DiMe’s Library of Digital Health Regulations and on the HAS and ANSM websites.
Engaging with national regulators
Health products submitted for marketing authorization in France undergo a structured review process with ANSM.
Additionally, reimbursement approval depends on the application pathway:
- LATM and LPPR: Evaluations should be completed within 180 days.
- PECAN: CNEDiMTS will issue an opinion within 60 days of application receipt; final validation by the French ministry of health can take an additional 30 days. If approved, manufacturers have 6 months to submit an application through LPPR for digital health tools intended for therapeutic purposes, or 9 months to submit a request under LATM for telemonitoring (remote) digital health tools.
If an application is missing information, the applicant will be notified and has 10 days to supply the requested information; if not furnished within that time period, the application can be deemed abandoned.
It is also important to note that in the PECAN pathway, manufacturers can submit the ANS technical certification application at the same time as the CNEDiMTS evaluation application. However, this is not the case for direct LPPR and LATM listing applications. In these two common-law pathways, manufacturers must first obtain their ANS certification before submitting the CNEDiMTS evaluation.
ANSM maintains general contact forms, including one for questions regarding medical devices. HAS does not maintain an online contact form, but can be contacted via mail or telephone. ANS can also be reached with questions.
ANSM has a standardized, mandatory process for requesting scientific advice for medical devices that incorporate a medicinal product and provides reference documents and contact details for initiating the request.
HAS offers pre-submission meetings for manufacturers as well. The agency provides an overview of the process for requesting a pre-submission meeting for medical devices prior to submitting to LPPR, LATM, or PECAN; further details are available in the PDF guide. If questions are simple enough, they may be answered by email or phone call; more complex situations may result in a meeting. These meetings must take place before the submission of a dossier.
ANS offers industry support through Ségur Support for authorized users (account creation).
Conformity assessments and CE marks for medical devices are administered by notified bodies. General information regarding obtaining a CE mark is available from the European Commission, as well as additional information and guidance documents for manufacturers related to the MDR legislation.
The European Commission additionally provides a list of country-specific contacts for obtaining a CE mark, as well as a list of current notified bodies, competent authorities, and other pertinent contacts for EU member states (including France). There are currently two approved notified bodies in France: GMED, and Afnor Certification.
ANSM maintains a detailed resource describing steps for manufacturers of various products as well as a dedicated page for medical device regulation. Generally, applications must be submitted electronically through Common European Submission Portal (CESP); specific submission information is described in this guide.
Submissions to LATM, LPPR, and PECAN, administered by HAS and CNEDIMTS, must be sent electronically through HAS Sésame. The application will be submitted to both agencies simultaneously and must also be submitted to the relevant national professional council(s). More information may be found in the application guide for each pathway: LATM, LPPR, PECAN.
After submission of a dossier, applicants will be assigned a contact at HAS for all communication. CNEDiMTS does not answer application inquiries.
For digital health products, applications must include a certificate of conformity with the security and interoperability standards (Public Health Code Article L. 1470-5). This certificate is conferred by ANS. An application may be submitted through the CONVERGENCE platform; please refer to the ANS resource for regulations, application procedures, and portal access.
Regulatory texts and additional information pertaining to MDR is provided by the European Commission (EC); the EC also provides periodic information sessions on pertinent topics.
ANSM provides comprehensive online resources for manufacturers, including regulatory documents, process information, and periodic information sessions.
HAS offers online resource guides and the opportunity for pre-submission meetings.
ANS also offers online resource guides and specialized support for industry through Ségur Support.
Glossary of terms
-
Actual clinical benefit (ACB)
-
ANS compliance
-
CE mark
-
Clinical added value (CAV)
-
Clinical benefit
-
Conformity assessment
-
Connected medical devices (CMDs)
-
EU medical device regulation (MDR)
-
Forfait Innovation
-
General data protection regulation (GDPR)
-
General product safety regulation (GPSR)
-
Medical device [MDR definition]
-
Notified Bodies
-
Actual clinical benefit (ACB)
Assessed for each indication of a product, and for population groups as applicable, based on 2 criteria:
- The benefit of the product with regard to its therapeutic or diagnostic effect or its effect in compensating for disability, as well as its adverse effects or risks related to its use and its role in the therapeutic strategy considering other available therapies; and
- The product’s expected public health benefit, in particular its impact on the health of the population, in terms of mortality, morbidity, and quality of life; its ability to meet a therapeutic need with regard to the severity of the condition or disability; and its impact on the healthcare system and on public health policies or programs.
Source: HAS
-
ANS compliance
The cybersecurity and interoperability standards set forth in Public Health Code Article L. 1470-5 to which all digital medical devices must demonstrate compliance. Applications for a compliance certificate are administered through the Agence de Numérique en Santé [Digital Health Agency; ANS].
Source: ANS
-
CE mark
A marking by which a manufacturer indicates that a device is in compliance with the applicable requirements set out in the EU MDR and other applicable European Union harmonization legislation.
Source: EU MDR
-
Clinical added value (CAV)
The assessment of the medical device compared to a comparable product, procedure, or service or to a group of comparable procedures, products, or services considered as standard according to the current data of science and subject or not subject to reimbursement. The CAV may be classified as major (I), important (II), moderate (III), minor (IV), or absent (V).
Source: HAS
-
Clinical benefit
The positive impact of a device on the health of an individual, expressed in terms of a meaningful, measurable, patient-relevant clinical outcome(s), including outcome(s) related to diagnosis, or a positive impact on patient management or public health.
Source: EU MDR
-
Conformity assessment
The process of determining whether the requirements of the EU MDR relating to a device have been fulfilled.
Source: EU MDR
-
Connected medical devices (CMDs)
An alternative term for DHTs. CMDs eligible for evaluation by the CNEDiMTS meet the following four criteria:
- They are intended for use for medical purposes, their end-use implying they are CE-marked.
- They are for individual use (implanted or used by the patient themselves).
- They have a telecommunication function.
- The company has submitted an application for reimbursement by national solidarity.
Source: HAS
-
EU medical device regulation (MDR)
Established by Regulation (EU) 2017/745 (EU MDR). The legislation governing the placing on the market, making available on the market, or putting into service medical devices for human use and accessories for such devices in the [European] Union. The Regulation also applies to clinical investigations concerning such medical devices and accessories conducted in the Union.
Source: EU MDR
-
Forfait Innovation
A fast-track program created to support innovative medical technologies. Eligible medical devices must be innovative and likely to provide significant health benefits or reduce healthcare expenses. In order to be reimbursed by the Forfait Innovation program, sponsors must have a protocol ready for a clinical trial or a health economic outcome study.
Source: HAS
-
General data protection regulation (GDPR)
EU regulation that governs how personal data of individuals in the EU is collected, processed, and protected. It aims to give individuals greater control over their personal information and imposes strict obligations on organizations to ensure data privacy, security, and transparency. GDPR applies to organizations that collect or target data related to people in the European Union (EU), even if the organization is not located in the EU.
Source: GDPR.EU
-
General product safety regulation (GPSR)
EU Legislation governing basic safety regulations for non-food products and all sales channels. Explicitly includes regulation of new and developing technologies.
Source: European Commission
-
Medical device [MDR definition]
“Any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease; diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability; investigation, replacement or modification of the anatomy or of a physiological or pathological process or state; providing information by means of in vitro examination of specimens derived from the human body, including organ, blood and tissue donations; and which does not achieve its principal intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its function by such means.” Devices for the control or support of conception and products specifically intended for the cleaning, disinfection, or sterilization of medical devices are also considered medical devices.
Source: EU MDR, Article 2
-
Notified Bodies
A conformity assessment body (a body that performs third-party conformity assessment activities including calibration, testing, certification, and inspection) designated in accordance with the EU MDR.
Source: EU MDR
Acknowledgements
DiMe thanks the following organizations and individuals for reviewing components of the France National Companion Guide, Flowchart, and Snapshot:
- Complear Health
- Digital Therapeutics Alliance
- Dario Motti, confinis ag
- Stéphane Tholander, Agora Health
- Hélène Viatgé, Agora Health













