

Germany National Companion Guide
Pursue DHT market access in Germany
Feedback
These pathways are constantly evolving. Work with us to keep them up to date by providing suggestions and feedback.
In Germany, DHT product regulatory authorization, value assessment, and pricing and reimbursement are conducted at the regional and national levels. However, developers often pursue patient access infrastructure via local routes.
Use the resources below to explore pathways for scalability and to start planning your regulatory strategy to bring your DHT to market in Germany.
What is a policy “full-stack”?
A policy “full-stack” provides a structured approach for integrating digital health technologies (DHT) into national healthcare systems.
Components of a comprehensive national policy for DHTs
Regulatory authorization
Product value assessment
Patient access infrastructure
Pricing & reimbursement
Read more about the core elements of a national policy “full-stack” approach.
This guide reflects current information as of 15 October 2024. National regulations and practices are subject to change and evolve, so developers are encouraged to regularly consult relevant agency websites for the latest information.
Explore DHT patient access and product scalability pathways
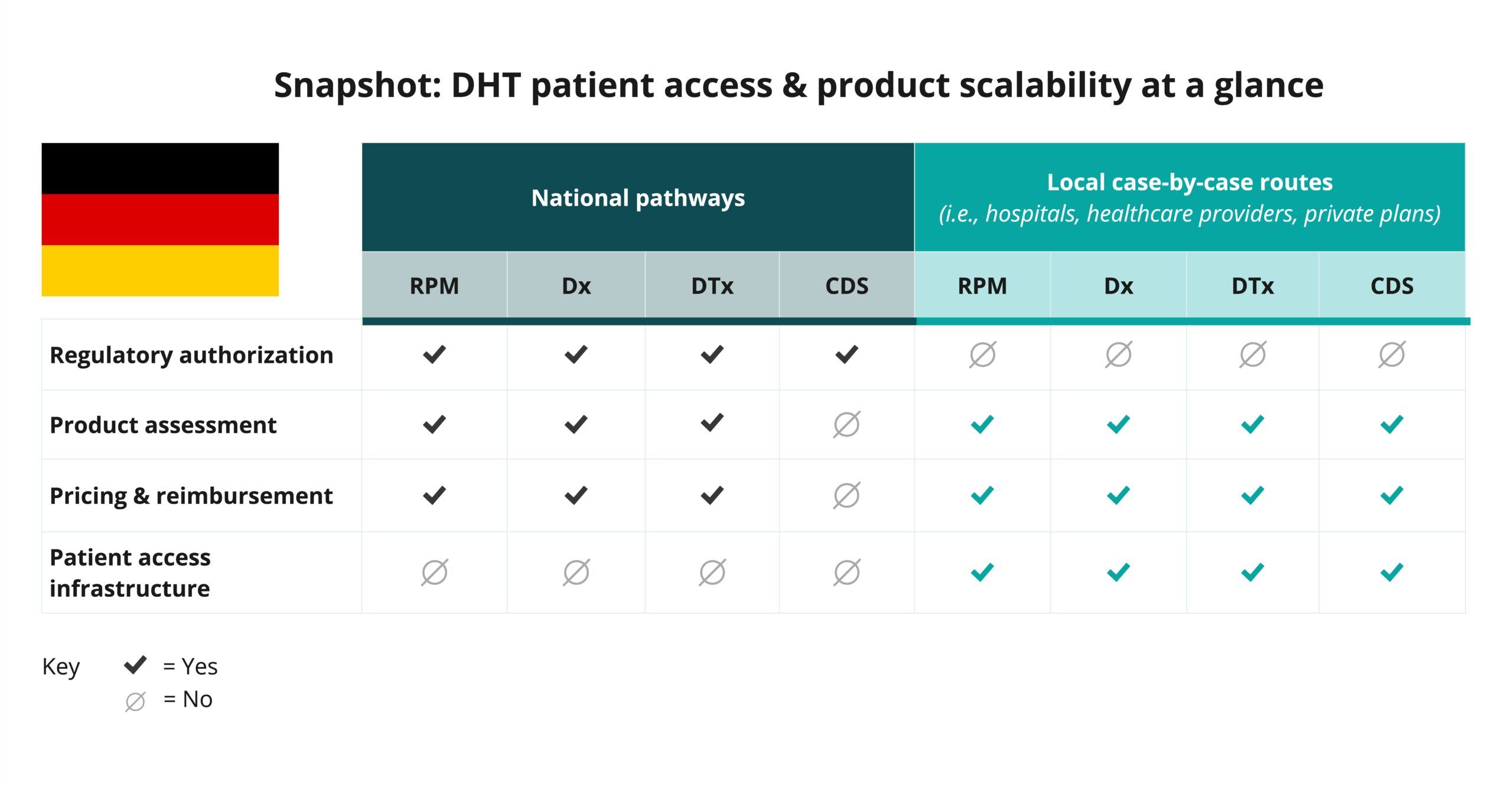
Begin your assessment of pathways in Germany by reviewing this snapshot, which offers insights into the potential patient access and product scalability pathways available for remote patient monitoring (RPM), digital diagnostic (Dx), digital therapeutics (DTx), and clinical decision support (CDS) products.
Navigate national pathways for DHT patient access
And then, use this flowchart to help you navigate Germany’s national pathways for DHT market access, guiding you through key steps in regulatory review, product assessment, pricing, reimbursement, and patient access. Aligned with the four core elements of a national policy “full-stack,” it focuses on national pathways and does not provide detailed insights on local routes.
As you move through the flowchart, you’ll see where developers need to generate or present clinical evidence to demonstrate each product’s safety, efficacy, and impact. “Clinical evidence evaluation” refers to a review of published data, while “clinical investigation” highlights when you may need to conduct a study to verify your product’s safety and performance.
Use this flowchart to stay informed at each stage and understand what’s expected to bring DHTs to patients in Germany.
This flowchart:
- Provides a sample of important steps along the pathway to patient access—not including post-market assessment pathways—and is subject to change. Other exclusions may exist.
- References DHT products that qualify as a medical device. DHT products that are not recognized as medical devices are not represented in this flowchart.
- Focuses primarily on national pathways and does not provide detailed insights on local case-by-case routes.
- Identifies certain opportunities to generate clinical evidence evaluations and investigations but does not incorporate health economic outcomes research (HEOR).
- Does not include specific timelines and is not intended to indicate how long or when steps are conducted.
National policy “full-stack” components
As indicated in the snapshot, DHT product regulatory authorization, value assessment, and pricing and reimbursement in Germany are conducted at the regional and national levels.
Click below for more insights
- The marketing of medical devices in EU member states is now governed by the EU Medical Device Regulation (MDR) (Regulation 2017/745), which was first enacted on 26 May 2017 and became mandatory for medical devices in Germany beginning 26 May 2020.
- General medical device classification requirements for the EU are set out in Annex VIII of Regulation 2017/745. Per the regulation, “Software, which drives a device or influences the use of a device, shall fall within the same class as the device. If the software is independent of any other device, it shall be classified in its own right.”
- In Germany, products such as remote patient monitoring (RPM), digital diagnostics (Dx), and digital therapeutics (DTx) products that qualify as medical devices may be classified into one of four categories: Class I, IIa, IIb, or III. Clinical decision support (CDS) products are not typically incorporated into national pathways.
- Germany’s Digital Healthcare Act (Digitale Gesundheitsanwendungen (DiGA)) was implemented on 19 December 2019, with the first DiGA products approved for use in October 2020. DiGA products:
- Are medical devices classified under risk Class I, IIa, or IIb.
- Must be prescribed by a physician or psychotherapist and address conditions indicated in ICD-10.
- Can qualify for permanent listing or preliminary/provisional listing for a typical period of 12 months.
- Are covered by the national statutory health insurance.
- The Digital-Gesetz (DigiG) came into effect in March 2024 and introduced significant changes to the DiGA process, including:
- Extending DiGA to incorporate:
- Risk Class IIb medical devices.
- Diagnostic DiGA, following extensive studies to prove the diagnostic sensitivity and specificity of the application.
- (Telemedical) monitoring products that:
- Not only support decisions made by the service providers, but also contain concrete recommendations for the patients.
- Generate individualized feedback based on patient input or the recording of sensor data that is explicitly aimed at the patients and their current medical situation and can be understood and implemented by them.
- Are implemented in connection with contract medical services carried out by registered physicians or psychotherapists.
- DiGA products that are designed to pair with an originator medicine must also be compatible with all available generic versions of the medicine, in addition to being updated when further generics become available.
- Extending DiGA to incorporate:
- Additionally, Germany’s Ordinance on the Eligibility for Reimbursement of Digital Nursing Applications (DiPAV) was published on 6 October 2022, creating a pathway for digital nursing applications (Digitale Pflegeanwendungen, DiPA) to be used in outpatient care settings. DiPA products are:
- Able, but not required to be, a medical device.
- Based on a patient’s need for care, as opposed to a specific ICD-10 code.
- Able to apply only for permanent inclusion in the directory and not preliminary/provisional listing.
- Covered by social care insurance, limited to 50 euros per month.
- A digital application can be listed in both DiPA and DiGA directories. As of 18 October 2024, 54 DiGA products are listed in the directory. No DiPA products are currently listed.
- A DiGA cannot be provisionally listed for more than 24 months, which includes the BfArM evaluation period.
- From 1 January 2026, the permanent remuneration amount for a DiGA will be at least 20% performance-related. The details of the transfer have yet to be agreed upon between the National Association of Health Insurance Funds and the manufacturers’ associations.
- Momentum is building towards integrating DiGA data with the national electronic patient record and e-prescribing system. In the interim, patients can access DiGA products either via prescription from their doctor or directly through the health insurance fund if they have a confirmed diagnosis of the indication the DiGA product is approved for.
- As a rule, health insurance companies should send the insured person the activation code for the relevant DiGA product within 2 working days of receiving the prescription. Exceeding this period is permitted only for serious reasons (e.g., in the event of serious technical problems when generating the activation code by the health insurance company).
- BfArM has no influence on the implementation of the legal obligation by the health insurance companies.
Curated overview of clinical evidence requirements and practices
To achieve a Conformité Européene (CE) mark via a notified body at the European level, medical devices must pass a conformity assessment, which certifies that the product meets legal requirements, is safe, and performs as intended.
Clinical evidence generated for applications to the DiGA and DiPA programs is typically conducted in Germany. If the systematic data collection did not take place in Germany, it must be plausibly demonstrated that the care situation in the country where the data was generated is comparable to that in Germany (i.e., comparable treatment guidelines, healthcare systems, patient access to the healthcare system, patient characteristics).
Click below for more insights
For the DiGA program, manufacturers must demonstrate that their product either can achieve (for provisional listing) or has already achieved (for permanent listing) positive healthcare effects (pVE). The required proof of efficacy must be based on a superiority study, where the DiGA shows better outcomes compared to its non-use in the context of usual care. The study’s endpoints and design must be carefully selected to provide evidence of the claimed pVE.
Conducting a randomized controlled trial (RCT) is not always required, but can simplify study planning and implementation. All DiGA products included in the directory have conducted an RCT (for permanent listing) or have planned an RCT as a proof-of-concept study (provisional listing). It is possible to combine clinical studies with real-world data (RWD) (e.g., if certain subgroups are not representative in the trial study) in order to provide proof of efficacy.
The most typical study designs for provisional listing include controlled intra-individual comparisons, RCTs with previous versions of the DiGA product, interim evaluations from RCTs, and pilot RCTs.
Legal provisions for the permanent listing of risk Class I and IIa products include:
- Comparative study showing the superiority of use over non-use.
- Retrospective comparative studies.
- Alternatively prospective comparative studies.
- A methodological approach that is appropriate to the positive healthcare effect.
- Analysis of non-use, which can be non-treatment or treatment without the DiGA product.
- Use of a comparator that corresponds to the reality of care, but can also be a DiGA product that is already permanently included.
- Submission of a prospective comparative study if there is no suitable data for retrospective comparisons or if sufficient comparability between the groups cannot be established.
Legal provisions for the permanent listing of risk Class IIb products include:
- Submission of a prospective comparative study to prove the medical benefit.
- Demonstration of patient-relevant improvement of structure and processes (pSVV), in addition to the medical benefit.
A more complete listing of resources may be found in DiMe’s Library of Digital Health Regulations and on BfArM’s website.
Engaging with national regulators
Medical devices in Germany are regulated by the EU Medical Device Regulation (MDR) legislation. MDR governs the approval and marketing for medical devices in EU member states.
Germany’s competent authority and national regulatory agency is the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM). It is responsible for monitoring and ensuring the safety and quality of medicinal products and medical devices in Germany.
Regarding reimbursement, the National Association of Statutory Health Insurance Funds serves as the central federal association for health insurance funds. Additionally, certain reimbursements may also be administered by long-term social care insurance (Pflegeversicherung, SPV).
European level: In order to market a medical device in Europe, a company must obtain a CE mark. For Class I medical devices, the manufacturer may carry out their own assessment; for higher-risk devices, a notified body chosen by the manufacturer must complete the conformity assessment. The European Commission provides resources regarding obtaining a CE mark, as well as additional information and guidance documents for manufacturers with regard to the MDR legislation.
Digital health products (DiGA and DiPA): Specific to digital health applications, Germany has created the DiGA pathway and provides a full guide to the application and approval process. Digital medical devices of Class I, IIa, and IIb that meet specific requirements may be submitted through this pathway for evaluation by BfArM and possible inclusion in the DiGA directory and price negotiation. Digital health applications for use in long-term care homes may be submitted through the DiPA pathway (guide available in German; English version forthcoming).
Medical devices: General information regarding medicinal products and medical devices in Germany is provided by BfArM. Conformity assessment, reporting, and notification requirements and procedures are governed under the Medical Device Law Implementation Act (Medizinprodukterecht-Durchführungsgesetz, MPDG) and the Medical Devices Act (Medizinproduktegesetz, MPG). BfArM is not involved in CE marking conformity assessments. Medical device approval is administered at the state level; refer to this list for state-level authorities and which product classes they handle [in German]. Companies should refer to the authority responsible for the state in which their company or distributor is located.
In the event of a disagreement regarding classification, regulation, etc., of a medical device between a company and a notified body, the company may submit an informal application to BfArM in accordance with S6 paragraph 1 of the MPDG. Or, alternatively, the appropriate state authority (Landesbehörde) may submit an application to BfArM in accordance with S6 paragraph 2 of the MPDG. Such applications may be submitted via email to mp-klar@bfarm.de. See instructions and details. Note that only the state authorities (Länder), and not BfArM, are authorized to make decisions regarding product marketability in Germany.
For digital health applications submitted through DiGA, evidence evaluation and a decision regarding preliminary admission will be rendered within 3 months of application; once granted preliminary approval, full evaluation and approval/rejection for the DiGA directory takes approximately 1 year. For digital health nursing applications submitted through DiPA, a decision regarding inclusion in the DiPA directory will be rendered within 3 months of application.
Most medical devices are regulated through state-level authorities (Länder); companies should refer to the appropriate authority based on their location.
Questions specific to DiGA may be submitted to diga@bfarm.de (contact also available by telephone). Questions specific to DiPA may be submitted to dipa@bfarm.de (or telephone).
BfArM may be contacted for general questions; please refer to the appropriate contacts by category. The agency also provides contact information for state-level authorities.
- BfArM provides pre-submission support through the Innovation Office. Two broad categories of support meetings are offered:
- Kick-off meetings, which are approximately 1 hour long and address general questions ahead of application or early in the application process; and
Scientific advice meetings, which are ~1.5 hours, provide more in-depth discussion, and are suitable for addressing more product-specific concerns for companies preparing applications.
Companies submitting through DiGA or DiPA may seek specific advice for those pathways through these mechanisms. Contact information and details regarding preparation for these meetings is available in this guide.
DiGA and DiPA: Applications must be submitted digitally; instructions available online (DiGA; DiPA).
Medical device regulation in Germany: Products must be registered with the appropriate state-level authority (determined by company location). Please refer to the contact information and details for specifics.
EU regulation of medical devices: All medical devices marketed in Germany must undergo conformity assessments and receive CE marks as prescribed by the MDR. Conformity assessments and CE marks for medical devices are administered by country-specific notified bodies. General information regarding obtaining a CE mark is available from the European Commission, as well as additional information and guidance documents for manufacturers with regard to the MDR legislation.
The European Commission additionally provides a list of country-specific contacts for obtaining a CE mark, as well as a list of current notified bodies, competent authorities, and other pertinent contacts for EU member states (including Germany).
DiGA and DiPA: Information and support specific to DiGA and DiPA applications are provided through the BfArM Innovation Office as detailed above.
Medical device approval in Germany: Information regarding general medical device approval is available through the BfArM website. Companies may engage with the Innovation Office as detailed above for further support. The Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) provides additional information specific to notified bodies and a contact form on their website.
EU medical device regulation: Regulatory texts and additional MDR information is provided by the European Commission; the EC also provides periodic information sessions on pertinent topics.
Glossary of terms
-
CE mark
A marking by which a manufacturer indicates that a device is in conformity with the applicable requirements set out in the EU MDR and other applicable Union harmonization legislation.
Source: EU MDR
-
Clinical benefit
The positive impact of a device on the health of an individual, expressed in the terms of a meaningful, measurable, patient-relevant clinical outcome(s), including outcome(s) related to diagnosis, or a positive impact on patient management or public health.
Source: EU MDR
-
Conformity assessment
The process demonstrating whether the requirements of the EU MDR relating to a device have been fulfilled.
Source: EU MDR
-
EU medical device regulation (MDR)
Established by Regulation (EU) 2017/745 (EU MDR). The legislation governing the placing on the market, making available on the market, or putting into service medical devices for human use and accessories for such devices in the [European] Union. The Regulation also applies to clinical investigations concerning such medical devices and accessories conducted in the Union.
Source: EU MDR
-
General data protection regulation (GDPR)
EU regulation that governs how personal data of individuals in the EU is collected, processed, and protected. It aims to give individuals greater control over their personal information and imposes strict obligations on organizations to ensure data privacy, security, and transparency. GDPR applies to organizations that collect or target data related to people in the European Union (EU), even if the organization is not located in the EU.
Source: GDPR.EU
-
General product safety regulation (GPSR)
EU Legislation governing basic safety regulations for non-food products and all sales channels. Explicitly includes regulation of new and developing technologies.
Source: European Commission
-
Medical device [MDR definition]
Any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes: diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease; diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability; investigation, replacement or modification of the anatomy or of a physiological or pathological process or state; providing information by means of in vitro examination of specimens derived from the human body, including organ, blood and tissue donations, and which does not achieve its principal intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its function by such means.” Devices for the control or support of conception and products specifically intended for the cleaning, disinfection, or sterilization of such devices are also considered medical devices.
Source: EU MDR, Article 2
-
Notified bodies
A conformity assessment body (a body that performs third-party conformity assessment activities including calibration, testing, certification and inspection) designated in accordance with the EU MDR.
Source: EU MDR
Acknowledgements
DiMe thanks the following organizations and individuals for reviewing components of the Germany National Companion Guide, Flowchart, and Snapshot:














