

England National Companion Guide
Pursue DHT market access in England
Feedback
These pathways are constantly evolving. Work with us to keep them up to date by providing suggestions and feedback.
In England, digital health technology (DHT) product regulatory authorization and value assessment are conducted at the national level. However, developers often pursue pricing and reimbursement and patient access infrastructure via a mixture of national and local routes.
Use the resources below to explore pathways for scalability and to start planning your regulatory strategy to bring your DHT to market in England.
What is a policy “full-stack”?
A policy “full-stack” provides a structured approach for integrating digital health technologies (DHT) into national healthcare systems.
Components of a comprehensive national policy for DHTs
Regulatory authorization
Product value assessment
Patient access infrastructure
Pricing & reimbursement
Read more about the core elements of a national policy “full-stack” approach.
This guide reflects current information as of 15 October 2024. National regulations and practices are subject to change and evolve, so developers are encouraged to regularly consult relevant agency websites for the latest information.
Explore DHT patient access and product scalability pathways
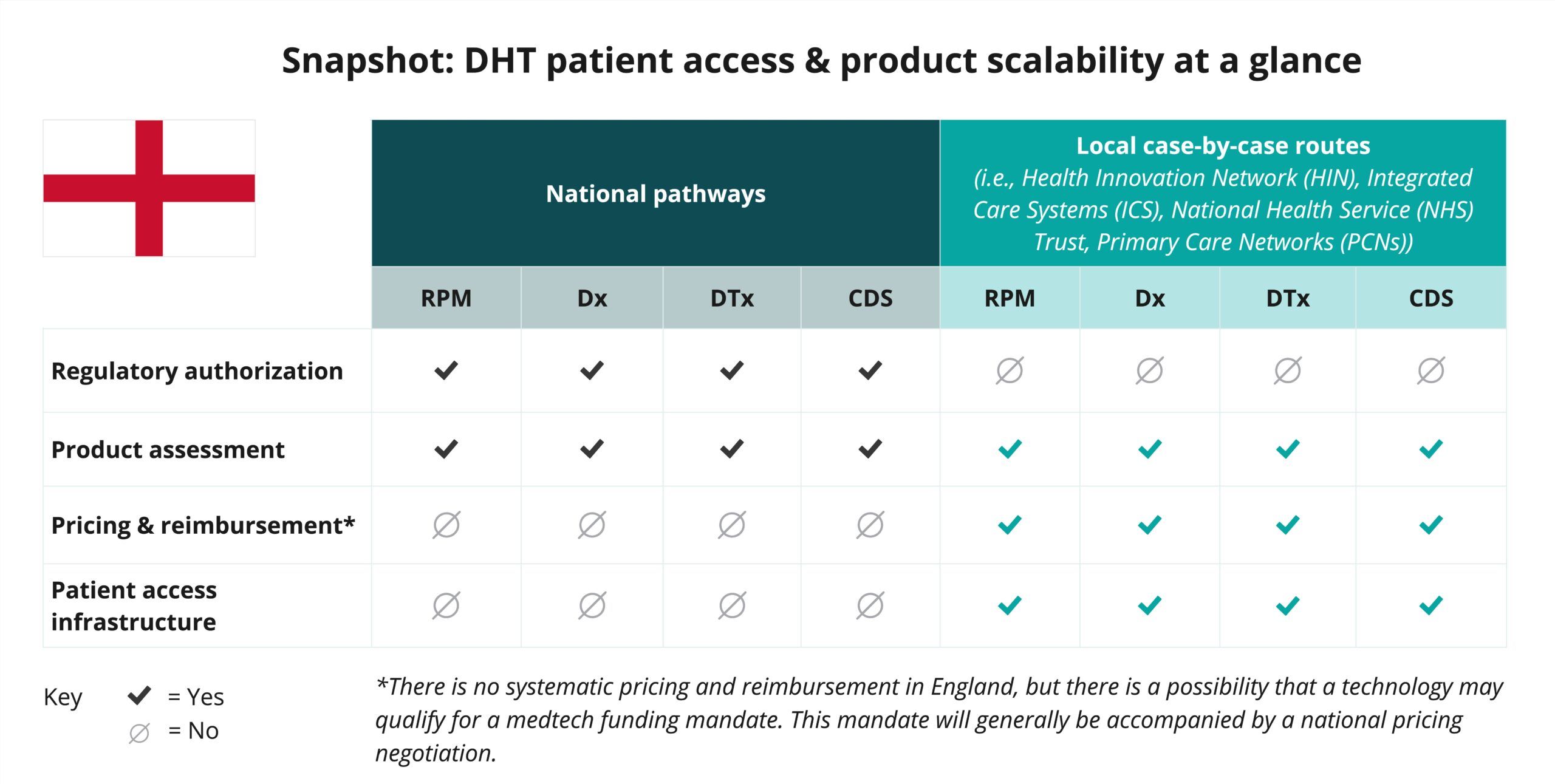
Begin your assessment of England’s routes to DHT patient access with this snapshot, which offers insights into the potential patient access and product scalability pathways available for remote patient monitoring (RPM), digital diagnostic (Dx), digital therapeutics (DTx), and clinical decision support (CDS) products.
Navigate national pathways for DHT patient access
And then, use this flowchart to help navigate England’s national pathways for DHT market access, guiding you through key steps in regulatory review, product assessment, pricing, reimbursement, and patient access. Aligned with the four core elements of a national policy “full-stack,” it focuses on national pathways and does not provide detailed insights on local routes.
As you move through the flowchart, you’ll see where developers need to generate or present clinical evidence to demonstrate each product’s safety, efficacy, and impact. “Clinical evidence evaluation” refers to a review of published data, while “clinical investigation” highlights when you may need to conduct a study to verify your product’s safety and performance.
This National Companion Guide provides product developers with comprehensive insights into navigating national pathways in England, as opposed to the entire United Kingdom (UK).
Use this flowchart to stay informed at each stage and understand what’s expected to bring DHTs to patients in England.
This flowchart:
- Provides a sample of important steps along the pathway to patient access—not including post-market assessment pathways—and is subject to change. Other exclusions may exist.
- References DHT products that qualify as a medical device. DHT products that are not recognized as medical devices are not represented in this flowchart.
- Focuses primarily on national pathways and does not provide detailed insights on local case-by-case routes.
- Identifies certain opportunities to generate clinical evidence evaluations and investigations but does not incorporate health economic outcomes research (HEOR).
- Does not include specific timelines and is not intended to indicate how long or when steps are conducted.
National policy “full-stack” components
As indicated in the snapshot, DHT product regulatory authorization and value assessment in England are conducted at the national level.
Click below for more insights
- In England, products such as remote patient monitoring (RPM), digital diagnostics (Dx), digital therapeutics (DTx), and clinical decision support (CDS) that qualify as medical devices can be classified into one of four categories: Class I, IIa, IIb, or III.
- Following the United Kingdom’s exit from the European Union (EU), the Medicines and Healthcare products Regulatory Agency (MHRA) invested in improving how medical devices are regulated in the UK. The Medicines and Medical Devices Act (2021) allows MHRA to amend the Medical Devices Regulations 2002 which govern medical devices in Great Britain; however, the UK medical device regulations remain largely unchanged and still heavily rely on the EU Medical Devices Directive (MDD). England’s regulatory frameworks continue to evolve.
- In addition to MHRA’s medical device regulatory authority in England, both the National Institute for Health and Care Excellence (NICE) and the National Health Service (NHS) provide national-level evaluation and assessment programs. However, most pricing, reimbursement, and patient access implementation occurs at the local level through entities like Health Innovation Networks (HINs), Integrated Care Systems (ICSs), NHS Trusts, and Primary Care Networks (PCNs).
- HINs support early-stage innovations and devices, in addition to offering regulatory support and guidance. Developers are therefore encouraged to engage early in their process.
- Programs such as NHS’s Innovation Accelerator and Test Bed Programme are designed to bring NHS organizations and industry partners together to test combinations of digital technologies in real-world settings.
- MHRA’s International Recognition Procedure (IRP) proposes steps for applicants that have already received authorization for the same product from one of MHRA’s specified reference regulators (RRs). This proposal may be subject to change.
Curated overview of clinical evidence requirements and practices
As part of the medical device essential requirements in England, clinical data is required for all medical devices to demonstrate claimed performance and safety. For some novel products, clinical investigations may be needed to validate claims.
Click below for more insights
A clinical evaluation, which involves looking at published clinical data to prove that the medical device works as intended and is safe to use, is not the same as a clinical investigation (CI). A CI may form part of a clinical evaluation. For example, if not enough pre-existing evidence exists to demonstrate that the device conforms to the essential requirements, a specifically designed CI may be necessary. In this case, manufacturers will need to notify MHRA in advance of the investigation. MEDDEV 2.7/4 includes guidance on deciding whether a CI is necessary. If a manufacturer intends to conduct a CI in the UK, they must inform MHRA at least 60 days before starting the investigation. This flowchart is useful for determining whether it is necessary to submit a study to MHRA in advance.
If using clinical data from other jurisdictions, it will be necessary to compare use cases, target populations, healthcare settings, and clinical care standards across both geographies. It is important to identify which evidence elements are, or are not, transferable across jurisdictions.
Additional insights related to clinical investigations of medical devices in England include:
- For clinical investigations that are also submitted to the FDA or other regulatory authorities, manufacturers should clearly indicate whether the UK and non-UK protocols are the same. If the protocols are not the same, the areas of difference should be referenced, and an explanation of the reasons for the differences should be provided. The objectives of a clinical investigation which is being conducted in the UK and also in a country or countries outside the UK may be wider than those required by the UK MDR 2002/EU MDR. Changes to protocol requested by other regulatory authorities should be copied to the MHRA. At such times, manufacturers should indicate whether the changes instigated by the non-UK regulatory authority will also be made to the UK protocol.
- The decision about selecting the most appropriate control group depends on the aims of the investigation. For some devices, it would only be possible to demonstrate claims adequately by comparison with a separate or untreated group. If control groups are necessary, they should be randomized and prospective, except in exceptional and justifiable circumstances. Pivotal/confirmatory studies should have a control where clinically relevant and appropriate to do so. For all studies, lack of a control group must be justified.
- Care should be taken in choosing endpoints to support the stated aims and objectives of the clinical investigation under normal conditions of use. Methods of supporting the demonstration of these endpoints should, as far as possible, be objective rather than subjective.
Developers should also be aware of compliance requirements related to product data and security, such as the Data Security and Protection Toolkit (DSPT), NHS Information Governance (GDPR and UK Protection Law compliance), and Clinical Risk Management DCB0160.
Engaging with national regulators
The AI and Digital Regulations Service is a good resource for requirements for medical devices and digital technologies in England (i.e., requirements for a quality management system (QMS), product technical documentation, data security, clinical safety). MHRA also maintains a library of guidance documents.
All medical devices must be registered with the MHRA before they can be placed on the market in Great Britain (England, Wales, and Scotland). If the manufacturer is based outside of the UK, they must appoint a single UK Responsible Person to take responsibility for all of their medical devices. The UK Responsible Person will then assume the responsibilities of the manufacturer in terms of registering the device with the MHRA.
For Class I medical devices, there is no specific review timeline, but instead a self-certification process. MHRA tends to register products following this route within a week of submission.
For Class IIa, IIb, and III medical devices, approval from a UK Approved Body (UKAB) can take one to two years. This timeline is impacted in part by the current backlog of device reviews of the closely related EU Notified Bodies; however, this backlog is expected to improve with the introduction of new conformity assessment routes by MHRA. Additionally, in May 2024, MHRA released a statement of policy intent for recognition by the UK of approvals of medical devices from international regulators.
The MHRA can be contacted via email with questions relating to product submissions. This consulting service may charge a fee in the future.
For companies seeking advice/support from NICE on the development of a health technology, NICE Advice operates separately from guidance production, offering fee-based advice and support around evidence generation or other challenges relating to market access. For general questions, there is an online contact form. Additionally, NICE provides a number of guidance documents on a variety of topics on their website.
MHRA does not have a broad pre-submission process for medical devices. This type of process is being developed in tandem with other relevant agencies through the Innovative Devices Access Pathway (IDAP) programme, but it is currently a limited access pilot program for a single cohort of devices.
Regulatory advice meetings: MHRA’s clinical investigations team can advise developers on planning clinical investigations.
Coordinated assessment pathway process: MHRA is collaborating with the Health Research Authority (HRA) on the coordinated assessment pathway, which involves the two organizations sharing information during the assessment of medical device clinical investigations. For this process, they require the MHRA clinical investigation application to be submitted first, and then the research ethics committee (REC) application to be submitted as soon as MHRA has confirmed the device’s application to be valid.
NICE Advice Service: Provides comprehensive pre-submission guidance. Resources include scientific advice meetings, the health economic model peer review service (META tool), “NICE surgery” meetings (1 hour meeting that is suitable for any point in the process), therapeutic landscape reviews (most suitable for early in the submission process), and system engagement meetings (most suitable for companies with Stage 3 clinical trial data who are preparing to formally submit).
UK Conformity Assessed (UKCA) marks are administered through the MHRA. Companies wanting to market a medical device in England must register the device with the MHRA through the Device Online Registration System (DORS).
The Digital Technology Assessment Criteria (DTAC) for health and social care was developed in response to developers and entities making buying and commissioning decisions that are looking for clear direction on how to build and buy good digital health technologies. It provides a way to review a product’s clinical safety, data protection, technical security, interoperability, and usability and accessibility standards. It also aligns with NICE’s Evidence Standards Framework for digital health technologies.
DTAC provides the national baseline criteria for DHTs entering and already used in the NHS and social care. For developers, it sets out what is expected for entry into the NHS and social care. Achieving the criteria laid out in the DTAC provides developers with a consistent way to present information to organizations buying and using digital health technologies. It does not imply that products will automatically be granted reimbursement or patient access if successfully completed.
Glossary of terms
-
Commissioning
-
Conformité Européene (CE) mark
-
Early value assessment
-
Evidence standards framework for digital health technologies
-
General data protection regulation (GDPR)
-
Guideline recommendation
-
Health Research Authority
-
Health technology assessment
-
Health technology evaluations
-
Incremental cost-effectiveness ratio
-
Marketing authorisation
-
Medical device
-
Medical technologies advisory committee
-
Medical Technologies Evaluation Programme
-
Commissioning
The process used by health services and local authorities to: identify the need for local services; assess this need against the services and resources available from public, private and voluntary organizations; decide priorities; and set up contracts and service agreements to buy services. As part of the commissioning process, services are regularly evaluated.
Source: NICE
-
Conformité Européene (CE) mark
A mandatory conformity mark on medical device products placed on the single market in the European Economic Area. It allows a manufacturer to sell their products within the European market, certifying that a product has met EU consumer safety, health, or environmental requirements.
Source: NICE
-
Early value assessment
An approach that allows rapid assessment of digital products, devices, and diagnostics for clinical effectiveness and value for money. This process allows the NHS and patients to benefit from these promising technologies while more evidence is collected.
Source: NICE
-
Evidence standards framework for digital health technologies
A framework that aims to ensure new digital health technologies are clinically effective and offer value to the health and care system. It guides developers through how to generate high-quality evidence.
Source: NICE
-
General data protection regulation (GDPR)
EU regulation that governs how personal data of individuals in the EU is collected, processed, and protected. It aims to give individuals greater control over their personal information and imposes strict obligations on organizations to ensure data privacy, security, and transparency. GDPR applies to organizations that collect or target data related to people in the European Union (EU), even if the organization is not located in the EU.
Source: GDPR.EU
-
Guideline recommendation
Specific advice in NICE guidelines on the care and services that are suitable for most people with a specific condition or need, or for particular groups or people in particular circumstances (for example, when being discharged from hospital). Recommendations may also cover ways to promote good health or prevent ill health, or how organizations and partnerships can improve the quality of care and services.
Source: NICE
-
Health Research Authority
Organization that protects the rights of patients, including by regulating the use of data collected within health and social care for research and product development.
Source: NICE
-
Health technology assessment
Independent research about the effectiveness, costs, and broader impact of healthcare (treatments and tests) for people who plan, provide, or receive care in the NHS.
Source: NICE
-
Health technology evaluations
Products assessed using the early value assessment approach are published as health technology evaluations.
Source: NICE
-
Incremental cost-effectiveness ratio
The incremental cost-effectiveness ratio (ICER) is the difference in the change in mean costs in the population of interest divided by the difference in the change in mean outcomes in the population of interest.
Source: NICE
-
Marketing authorisation
Previously known as a product license and granted to medicines that meet the standards of safety, quality, and efficacy set by a medicines regulator (for example, the Medicines and Healthcare products Regulatory Agency or the European Medicines Agency); is normally necessary before a regulated product can be prescribed or sold.
Source: NICE
-
Medical device
A medical device is any instrument, apparatus, appliance, software, material, or other article which is intended for human use that performs a medical purpose, such as diagnosing, monitoring, or treating a medical condition. These devices could be hardware, software, or appliances. The definition explicitly includes software, applications, and consumer devices that collect medical information.
Source: NHS England
-
Medical technologies advisory committee
An independent committee with 2 roles: selecting medical technologies for evaluation by other NICE guidance programs, and developing medical technologies guidance itself.
Source: NICE
-
Medical Technologies Evaluation Programme
A NICE program to identify medical technologies that could offer benefits to patients or the NHS. Companies notify NICE about possible topics. The medical technologies advisory committee selects products for evaluation. It may carry out the evaluation itself or refer the topic to be evaluated by another NICE program, usually technology appraisals or interventional procedures or diagnostics, or sometimes guidelines.
Source: NICE
Acknowledgements
DiMe thanks the following organizations and individuals for reviewing components of the England National Companion Guide, Flowchart, and Snapshot:
















