Confidently navigate the U.S. digital health regulatory landscape
Find your pathway to optimize your product and business decision-making.

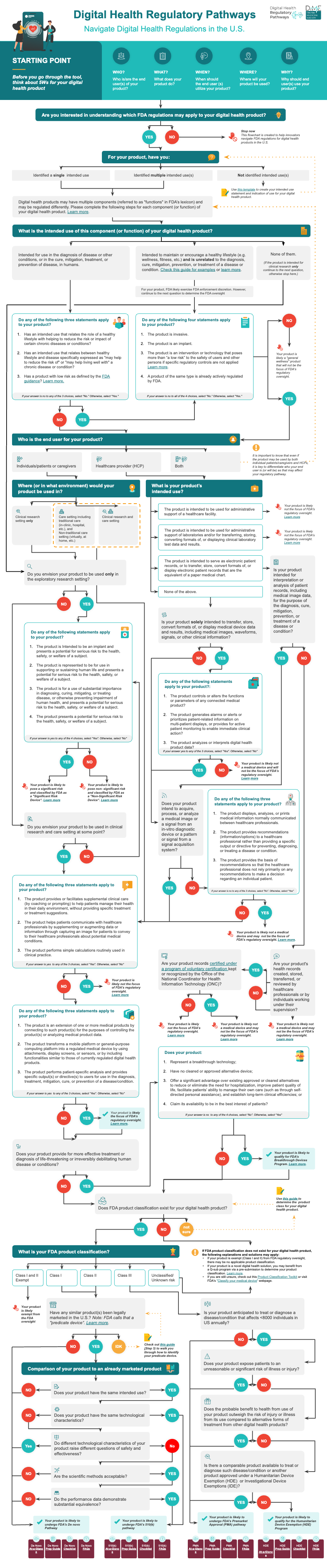
Identify Your Pathway with the RegPath Decision Tool
Use this tool to discover an approach, along with relevant resources, to pursuing a regulatory pathway that is specific to your particular situation and digital health tool.
This tool has not been optimized for mobile. Please visit this link on a desktop.














The FDA also actively participated in the development of these resources. Please note that the tools developed as a part of DiMe’s Digital Health Regulatory Pathways project are not intended to replace any written law, regulations, formal FDA device determination for your product or to be considered a formal recommendation on behalf of the FDA or any partners of the project. They are meant to support innovators in the development of high-quality, impactful, and trustworthy digital health products.
Library of Digital Health Regulations
Innovators and industry stakeholders across the healthcare system can use this centralized Library of Digital Health Regulations to keep up with the latest regulations and guidances applicable to digital health.
See something we missed?
Identify
Use the RegPath tool and other resources to navigate and identify your regulatory pathway.
Build
Create and integrate a fit-for-purpose regulatory strategy into your business plan for commercial success.
Interact
Ongoing, interact with regulators to develop digital health products that best meet your goals and patients’ needs.