Celebrating the Fourth Anniversary of the Library of Digital Endpoints!
Introducing the Library of Digital Endpoints
This month, the Digital Health Measurement Collaborative Community (DATAcc) by the Digital Medicine Society (DiMe) is thrilled to be celebrating the fourth anniversary of the Library of Digital Endpoints. It is a pioneering library with a comprehensive list of digitally-collected endpoints used in industry-sponsored studies, sourced from credible clinical trial registries and crowdsourced submissions.
Library growth
When we first launched the Library of Digital Endpoints, collaborative science wasn’t yet on the cards, but through the library, we drove remarkable advances in the field by creating a centralized source for digital endpoints.
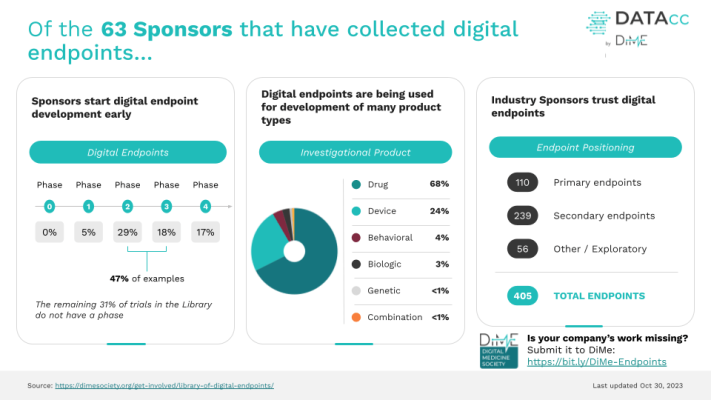
In 2019, 35 digital endpoints were being used in industry-sponsored trials. Today, we have cataloged 405 digital endpoints – a more than 10-fold increase in just four years – illustrating the immense growth, maturity, and increasing trust and adoption of digital measurement in clinical research.
In light of this rapid expansion, we formalized the eligibility criteria for the library to complement and support this growth, ensuring that the library’s content reflects digital endpoints most relevant to potential users.
Formalizing new eligibility criteria
Endpoints are eligible for inclusion if they meet the following criteria:
- The trial is listed on a reputable registry;
- The trial is industry-funded;
- The trial assesses a regulated intervention designed for screening, diagnosis, treatment, or disease prevention;
- The trial includes one or more safety and/or efficacy/effectiveness endpoints captured by a sensor-based digital health technology product.
| Endpoints are eligible if the trial: | Description |
| Is registered on a public clinical trials registry | The registry must be on the World Health Organization International Trials Registry Platform (WHO ICTRP) list of primary registries or ClinicalTrials.Gov which is a data provider to the World Health Organization. |
| Endpoints are eligible if the trial: | Description |
| Is funded by industry | Industry is defined as a for-profit entity, excluding government agencies, individuals, universities or other educational organizations, and registered non-profits. |
| Is interventional, with the intervention being a drug, biologic, genetic intervention, regulated diagnostic test, regulated medical device, regulated combination product, regulated behavioral intervention, regulated procedural/surgical intervention, or regulated radiation intervention | Interventional studies are defined as those in which participants are assigned prospectively to an intervention or interventions according to a protocol to evaluate the effect of the intervention(s) on biomedical or other health-related outcomes.A complete list of intervention types is available at ClinicalTrials.Gov. |
| Have a primary purpose listed as one of the following: treatment, prevention, diagnostic, or screening | A complete list of intervention types is available at ClinicalTrials.Gov. |
| Include at least one safety or efficacy/effectiveness endpoint captured by a sensor-based digital health technology | Safety endpoints are those reflecting side effects of the interventionEfficacy and effectiveness endpoints are those reflecting the performance of the intervention under controlled and real-world conditions, respectively.Sensor-based DHTs are defined as: Connected digital medicine products that process data captured by mobile sensors using algorithms to generate measures of behavioral and/or physiological function. |
*Note: The endpoints captured by the sensor-based digital health technology must assess the trial intervention’s safety or efficacy/effectiveness and not the digital health technology itself.
Help us continue to grow
We have also recently enhanced the library by adding more data for each endpoint and incorporated new filters that make the library more intuitive and even easier to use. We encourage you to help us maintain the incredible momentum we’ve seen to date and submit endpoints, and share how you’re using the library.
We would like to celebrate your organization if you have used the library to support and inform your work! Tell us how you’ve used the Library of Digital Endpoints by filling out a brief Resource in Action form, and we’d love to highlight how you and/or your organization are leveraging digital innovation to improve clinical research. You can submit endpoints directly to the library here.
- https://www.who.int/clinical-trials-registry-platform/network/primary-registries
- This entire definition has been adopted by the International Committee of Medical Journal Editors
- 42 CFR 11.10(a) “Interventional”
- See Section 8: https://prsinfo.clinicaltrials.gov/definitions.html
- See Section 7: https://prsinfo.clinicaltrials.gov/definitions.html
- DiMe Glossary definition, referencing the FDA Clinical Trials for Patient Engagement Advisory Committee: https://www.fda.gov/media/108378/download
- Per the V3 Framework: https://pubmed.ncbi.nlm.nih.gov/32337371/



