

Australia National Companion Guide
Pursue DHT market access in Australia
Feedback
These pathways are constantly evolving. Work with us to keep them up to date by providing suggestions and feedback.
In Australia, regulatory authorization for digital health technology (DHT) products is managed at the national level. However, developers often seek product value assessment, pricing, reimbursement, and patient access through local routes.
Use the resources below to explore pathways for scalability and to start planning your regulatory strategy to bring your DHT to market in Australia.
What is a policy “full-stack”?
A policy “full-stack” provides a structured approach for integrating digital health technologies (DHT) into national healthcare systems.
Components of a comprehensive national policy for DHTs
Regulatory authorization
Product value assessment
Patient access infrastructure
Pricing & reimbursement
Read more about the core elements of a national policy “full-stack” approach.
This guide reflects current information as of 15 October 2024. National regulations and practices are subject to change and evolve, so developers are encouraged to regularly consult relevant agency websites for the latest information.
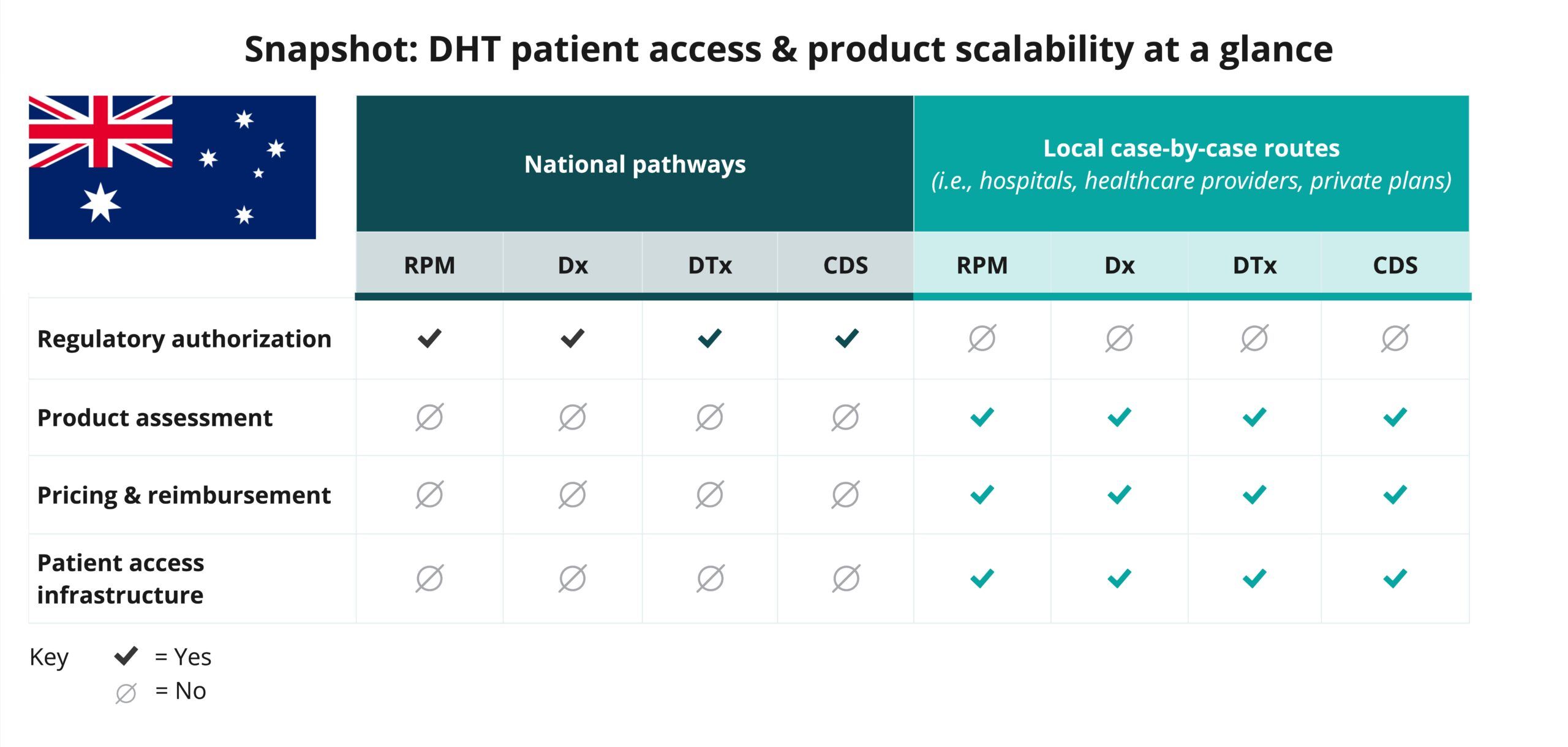
Explore DHT patient access and product scalability pathways
Begin your assessment of Australia’s routes to DHT patient access by reviewing this snapshot, which offers insights into the potential patient access and product scalability pathways available for remote patient monitoring (RPM), digital diagnostic (Dx), digital therapeutics (DTx), and clinical decision support (CDS) products.
Navigate national pathways for DHT patient access
And then, use this flowchart to help you navigate Australia’s national pathways for DHT patient access, guiding you through key steps in regulatory review, product assessment, pricing and reimbursement, and patient access processes. Aligned with the four core elements of a national policy “full-stack,” it focuses on national pathways and does not provide detailed insights on local routes.
As you move through the flowchart, you’ll see where developers need to generate or present clinical evidence to demonstrate each product’s safety, efficacy, and impact. “Clinical evidence evaluation” refers to a review of published data, while “clinical investigation” highlights when you may need to conduct a study to verify your product’s safety and performance.
Use this flowchart to stay informed at each stage and understand what’s expected to bring DHTs to patients in Australia.
This flowchart:
- Provides a sample of important steps along the pathway to patient access—not including post-market assessment pathways—and is subject to change. Other exclusions may exist.
- References DHT products that qualify as a medical device. DHT products that are not recognized as medical devices are not represented in this flowchart.
- Focuses primarily on national pathways and does not provide detailed insights on local case-by-case routes.
- Identifies certain opportunities to generate clinical evidence evaluations and investigations but does not incorporate health economic outcomes research (HEOR).
- Does not include specific timelines and is not intended to indicate how long or when steps are conducted.
National policy “full-stack” components
As indicated in the snapshot, DHT product regulatory authorization in Australia is conducted at the national level.
Click below for more insights
The Therapeutic Goods Administration (TGA) regulates medical devices in Australia, including software and mobile apps that meet the definition of a medical device. If a software product is a medical device, it must be included in the Australian Register of Therapeutic Goods (ARTG), unless it is exempt, before it can be legally supplied in Australia. Additional insights related to the regulatory authorization process in Australia include:
- The role of TGA spans the entire medical device lifecycle, from market authorization before a device is supplied, through to post-market monitoring and review of medical devices, including software-based medical devices, already on the market, to ensure these devices continue to meet regulatory requirements.
- Digital health technologies (DHTs) encompass a wide range of intended uses and risk levels=. In Australia, products such as remote patient monitoring (RPM), digital diagnostics (Dx), digital therapeutics (DTx), and clinical decision support (CDS) products that qualify as medical devices can be classified into one of four categories: Class I, IIa, IIb, or III.
- The majority of medical devices are supplied in Australia on the basis of European Union (EU) certification. The Australian classification rules for software-based medical devices are broadly aligned with the EU classifications.
- The Australian government amended the Therapeutic Goods (Medical Devices) Regulations 2002 on 25 February 2021. Changes took full effect on 1 November 2024. They are also undertaking additional reforms to strengthen the regulation of medical devices in Australia.
Curated overview of clinical evidence requirements and practices
TGA provides numerous resources on clinical trial requirements and guidelines. TGA additionally provides developers with insights on navigating overseas regulatory evidence options for a medical device application. A more complete listing of resources may be found on the TGA website and in DiMe’s Library of Digital Health Regulations.
Click below for more insights
- As part of TGA’s Essential Principles, clinical evidence is required for each device unless designated as an “excluded” device.
- Clinical evaluation of medical devices based on existing, established technologies and intended for an established use of the technology is most likely to rely on compliance with recognized standards, literature reviews, and/or clinical experience of comparable devices.
- High-risk devices, devices based on technologies where there is little or no experience, and devices that extend the intended purpose of an existing technology (i.e., a new clinical use) are most likely to require clinical investigation data.
- TGA is not prescriptive but provides detailed guidance (i.e., Australian clinical trial handbook, regulation essentials).
- It is important for manufacturers to always have clinical evidence “on hand” since TGA can request to review product evidence at any time.
- TGA is well aligned with requirements established in the European Union.
- Clinical evidence can be accepted from other jurisdictions. However, even if evidence is accepted from another jurisdiction, it may be reviewed or interpreted differently by TGA. When clinical investigation data is collected outside Australia:
- The study population and demographics must be relevant to Australia and the intended scope/use of the product in Australia.
- The investigation must comply with the principles of the Declaration of Helsinki (clause 8.4(5) of Part 8 of Schedule 3 of the MD Regulations).
- Trials must comply with the International Conference on Harmonisation (ICH) Guideline for Good Clinical Practice and ISO 14155:2020.
Transferability of clinical evidence from other jurisdictions for priority determination applications usually aligns with the guidance specified above for conformity assessment applications, noting that demonstration of unmet need and major clinical advantage should justify applicability of the clinical evidence to the Australian context.
Engaging with national regulators
The Therapeutic Goods Administration (TGA) is Australia’s government authority responsible for evaluating, assessing, and monitoring products defined as therapeutic goods. TGA regulates medicines, medical devices, and biologicals to help Australians stay healthy and safe.
The most important timelines to consider are Australia’s medical device application processing times.
The Essential Principles checklist helps medical device manufacturers prove compliance with the Essential Principles. This checklist aims to help:
- Identify the safety and performance requirements that apply to the device.
- Document a rationale for any of the safety and performance requirements that are not relevant.
- Summarize the evidence developers hold in support of each of the relevant safety and performance requirements.
Where applicable, developers may need to meet National Safety and Quality Digital Mental Health (NSQDMH) Standards.
Get in touch with TGA by phone, email or post.
- Business area contacts: For inquiries that relate to a certain business area, please see contact details.
- What TGA can help with: Inquiries about a medicine, a medical device, or other therapeutic good.
- What TGA cannot help with: TGA cannot answer questions unrelated to therapeutic goods. They also cannot respond to inquiries on researching or developing therapeutic goods, giving clinical advice to individuals, recommending products, or making decisions on subsidies.
In addition, in partnership with TGA, Australia’s national digital health initiative, ANDHealth, runs free one-on-one office hours to support companies in navigating TGA guidance.
Pre-submission meetings with TGA: Guidance for applicants on how to request and prepare for pre-submission meetings.
Scientific advice is not applicable to medical devices.
Submit an application for inclusion of a medical device. Different steps apply for different categories and types of devices.
The TGA Business Services (TBS) site allows industry partners to manage some therapeutic good registration applications and view and cancel their current entries on the ARTG. Sponsors can view, download, and print ARTG Register entries for their products and generate certificates online. Clients can also keep up to date with the latest TGA-wide and industry-specific news directly from the site.
Manufacturers intending to supply a medical device in Australia need to meet the definition of a Sponsor under the Therapeutic Goods Act 1989. These requirements include that manufacturers must be a recognized Australian-based legal entity.
Before developers apply to include a medical device in the ARTG, it is necessary that a relationship with the medical device manufacturer is established to:
- Obtain documentation/information required to demonstrate that the kind of medical device complies with the regulatory requirements in Australia.
- Provide documentation/information relating to the regulatory, technical, clinical, and safety aspects of the device that TGA may request at any time while companies are supplying the device in Australia.
- Advise TGA of any problems, safety alerts, or recalls that may arise with the use of the device.
Glossary of terms
- Active medical device for diagnosis
- Australian Register of Therapeutic Goods (ARTG)
- Clinical decision support software (CDSS)
- Conformity assessment
- Digital diagnostic
- Digital therapeutic (DTx)
- Essential Principles (EPs)
- Excluded products
- Exempt software
- Manufacturer
- Medical device
- Remote patient monitoring (RPM)
- Software as a medical device (SaMD)
- Software in a medical device (SiMD)
- Sponsor
Active medical device for diagnosis
An active medical device intended by the manufacturer to be used on a human being, either alone or in combination with another medical device, to supply information for the purpose of detecting, diagnosing, monitoring, or treating physiological conditions, states of health, illnesses or congenital deformities.
Australian Register of Therapeutic Goods (ARTG)
The ARTG is the public database of therapeutic goods that can be legally supplied in Australia.
Clinical decision support software (CDSS)
CDSS is software that can perform a broad range of functions that facilitate, support, and enable clinical practice. If the CDSS is exempt, it is not required to be registered in the ARTG. However, the following requirements still apply:
- Sponsors must notify the TGA of their exempt CDSS devices.
- Sponsors of exempt devices must ensure the devices meet the relevant essential principles for safety and performance of medical devices.
- The TGA can take regulatory action, such as a recall or issuing a hazard alert if there is a problem with the device.
- Sponsors must report adverse events to the TGA.
CDSS that meets the definition of a medical device must be included in the Australian Register of Therapeutic Goods (ARTG) unless otherwise exempt. Under the changes, an exemption has been introduced for some CDSS that is a medical device. Note: CDSS that does not meet the definition of a medical device, or is excluded, is not subject to regulation by the TGA.
Conformity assessment
The systematic examination of evidence generated, and procedures undertaken by the manufacturer, under requirements established by the Regulatory Authority, to determine that a medical device conforms to the Essential Principles.https://www.tga.gov.au/how-we-regulate/manufacturing/medical-devices/conformity-assessment/conformity-assessment-bodies/tga-conformity-assessment-certification/conformity-assessment-overview
Digital diagnostic
An active medical device intended by the manufacturer to be used on a human being, either alone or in combination with another medical device, to supply information for the purpose of detecting, diagnosing, monitoring, or treating physiological conditions, states of health, illnesses, or congenital deformities.
Source: TGA
Digital therapeutic (DTx)
Health software intended to treat or alleviate a disease, disorder, condition, or injury. It works by generating and delivering a medical intervention that has a demonstrated positive impact on a patient’s health. This definition aligns with the definition for DTx in an international standard Health Informatics – Personalized digital heath – Digital therapeutics health software systems (ISO/ TR 11147-2023).
DTx is a subset of software as a medical device (SaMD). TGA regulates DTx and other types of software and apps that meet the definition of a medical device, under the regulatory framework for software-based medical devices.
Source: TGA
Essential Principles (EPs)
Principles that set out requirements relating to device safety and performance and to which medical devices must comply. There are 6 general and 10 specific EPs.
Source: TGA
Excluded products
Products that are not medical devices and are not subject to any TGA regulatory requirements. Excluded products do not need to be included in the Australian Register of Therapeutic Goods (ARTG).
Source: TGA
Exempt software
Software that is a medical device but is not subject to all regulatory requirements.
Source: TGA
Manufacturer
Person or company taking legal responsibility for the manufacture of a medical device (the name on the label, or the name under which the product is supplied). The manufacturer of a medical device is responsible for the design, production, packaging, and labeling of the device, but does not necessarily need to perform these activities themselves.
Source: TGA
Medical device
Australian law defines a medical device as:
-
- Any instrument, apparatus, appliance, software, implant, reagent, material, or other article (whether used alone or in combination, and including the software necessary for its proper application) intended, by the person under whose name it is or is to be supplied, to be used for human beings for the purpose of one or more of the following:
- diagnosis, prevention, monitoring, prediction, prognosis, treatment, or alleviation of disease;
- diagnosis, monitoring, treatment, alleviation of or compensation for an injury or disability;
- investigation, replacement, or modification of the anatomy or of a physiological or pathological process or state;
control or support of conception; or - in vitro examination of a specimen derived from the human body for a specific medical purpose;
and that does not achieve its principal intended action in or on the human body by pharmacological, immunological, or metabolic means, but that may be assisted in its function by such means; or
- Any instrument, apparatus, appliance, software, implant, reagent, material, or other article specified under subsection (2A); or
- Any instrument, apparatus, appliance, software, implant, reagent, material, or other article that is included in a class of instruments, apparatuses, appliances, software, implants, reagents, materials, or other articles specified under subsection (2B); or
- An accessory to an instrument, apparatus, appliance, software, implant, reagent, material, or other article covered by paragraph (a), (b) or (c); or
- A system or procedure pack.
- Any instrument, apparatus, appliance, software, implant, reagent, material, or other article (whether used alone or in combination, and including the software necessary for its proper application) intended, by the person under whose name it is or is to be supplied, to be used for human beings for the purpose of one or more of the following:
Source: Federal Register of Legislation
Remote patient monitoring (RPM)
Active medical devices intended to provide information used to monitor the state or progression of a disease or condition of a person. Medical devices in this category process data in order to provide an output in the form of information, or parameters, that indicate the state of a disease or condition of a person. The data used as an input to such a device may include multiple or single sources and could consist of, for example, data provided by physiologic sensors, such as heart rate monitors, or data from other medical devices, such as those used in an intensive care unit.
Source: TGA
Software as a medical device (SaMD)
Software that can function on, for example, a laptop computer, smartphone or tablet, and has an intended purpose consistent with the definition of a medical device. This software could be any kind of software, including but not limited to: computer programs and applications, mobile apps, software as a service (cloud-based), websites, and browser-delivered products.
Software would generally be a medical device if it is intended to be used for:
- diagnosis, prevention, monitoring, prediction, prognosis, or treatment of a disease, injury, or disability,
- compensation for an injury or disability,
- investigation of the anatomy or of a physiological process, or
- to control conception.
Source: TGA
Software in a medical device (SiMD)
Software can be part of a device when it is integral to the functioning of that device. This software is usually supplied with the hardware device.
Source: TGA
Sponsor
Person or company legally responsible for supplying devices in Australia, or exporting medical devices out of Australia.
Source: TGA
Acknowledgements
DiMe thanks the following organizations and individuals for reviewing components of the Australia National Companion Guide, Flowchart, and Snapshot:
- ANDHealth, Australia’s National Digital Health Initiative
- Chris Boyd-Skinner, Digital Mental Health Consultant












