

China National Companion Guide
Pursue DHT market access in China
Feedback
These pathways are constantly evolving. Work with us to keep them up to date by providing suggestions and feedback.
In China, DHT product regulatory authorization is conducted at the national level for Class III products. However, developers often pursue regulatory authorization for Class II products, product value assessment, pricing and reimbursement, and patient access infrastructure via local routes.
Use the resources below to explore pathways for scalability and to start planning your regulatory strategy to bring your DHT to market in China.
What is a policy “full-stack”?
A policy “full-stack” provides a structured approach for integrating digital health technologies (DHT) into national healthcare systems.
Components of a comprehensive national policy for DHTs
Regulatory authorization
Product value assessment
Patient access infrastructure
Pricing & reimbursement
Read more about the core elements of a national policy “full-stack” approach.
This guide reflects current information as of 15 October 2024. National regulations and practices are subject to change and evolve, so developers are encouraged to regularly consult relevant agency websites for the latest information.
Explore DHT patient access and product scalability pathways
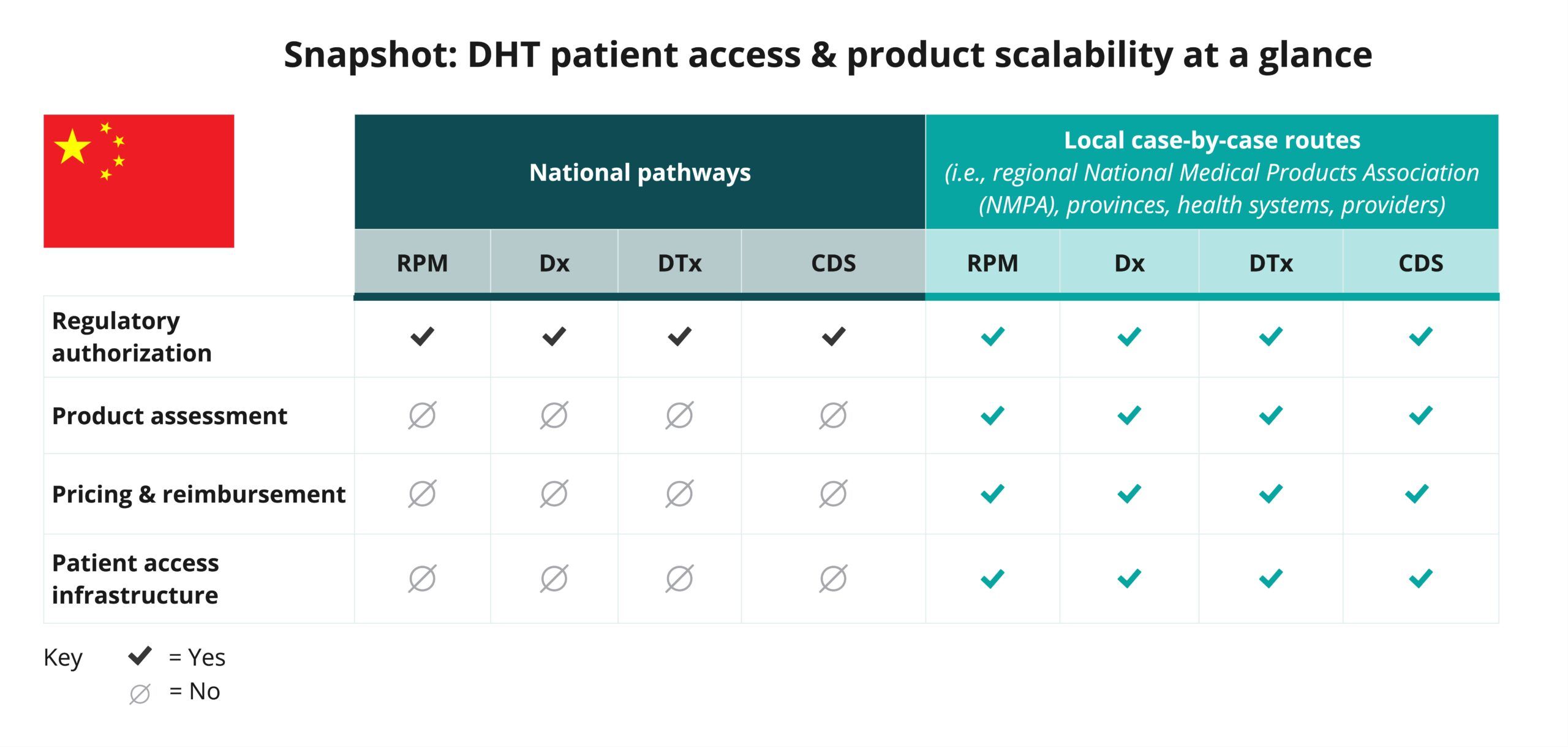
Begin your assessment of China’s pathways by reviewing this snapshot, which offers insights into the potential patient access and product scalability pathways available in China for remote patient monitoring (RPM), digital diagnostic (Dx), digital therapeutics (DTx), and clinical decision support (CDS) products.
Navigate national pathways for DHT patient access
And then, use this flowchart to help you navigate China’s national pathways for DHT market access, guiding you through key steps in regulatory review, product assessment, pricing, reimbursement, and patient access. Aligned with the four core elements of a national policy “full-stack,” it focuses on national pathways and does not provide detailed insights on local routes.
As you move through the flowchart, you’ll see where developers need to generate or present clinical evidence to demonstrate each product’s safety, efficacy, and impact. “Clinical evidence evaluation” refers to a review of published data, while “clinical investigation” highlights when you may need to conduct a study to verify your product’s safety and performance.
Use this flowchart to stay informed at each stage and understand what’s expected to bring DHTs to patients in China.
This flowchart:
- Provides a sample of important steps along the pathway to patient access—not including post-market assessment pathways—and is subject to change. Other exclusions may exist.
- References DHT products that qualify as a medical device. DHT products that are not recognized as medical devices are not represented in this flowchart.
- Focuses primarily on national pathways and does not provide detailed insights on local case-by-case routes.
- Identifies certain opportunities to generate clinical evidence evaluations and investigations but does not incorporate health economic outcomes research (HEOR).
- Does not include specific timelines and is not intended to indicate how long or when steps are conducted.
National policy “full-stack” components
As indicated in the snapshot, DHT product regulatory authorization for imported and Class III medical devices in China is conducted at the national level.
Click below for more insights
- Digital health technologies (DHTs) encompass a wide range of intended uses and risk levels. In China, products such as remote patient monitoring (RPM), digital diagnostics (Dx), digital therapeutics (DTx), and clinical decision support (CDS) products that qualify as medical devices can be classified as Class II or III of the three available product categories: Class I, II, or III.
- The Chinese Center for the Evaluation of Medical Devices (CMDE) is in charge of the technical review of medical devices, and the National Medical Products Administration (NMPA) provides the final approval and authorization.
- Software as a Medical Device (SaMD) products are categorized into six categories: treatment planning software, image processing software, data processing software, decision support software, in vitro diagnostic software, and other software.
- The incorporation of artificial intelligence/machine learning (AI/ML) in medical devices can impact the classification of a product and typically results in a Class III designation.
The risk degree of a medical device can be comprehensively determined according to the intended purpose, structural characteristics, pattern of use, and status of use, as well as whether the device is in contact with the body.
Curated overview of clinical evidence requirements and practices
In line with China’s Provisions for Medical Device Registration and Filing, clinical data is required for all medical devices in China, with the following limited exceptions:
- Where the functional mechanism of the device is definite, the design is finalized, the manufacturing process is well-established, the marketed predicate device has been in clinical use for years, no serious adverse events are recorded, and its regular intended use is not changed.
- Where the safety and effectiveness of the medical device can be proven through non-clinical evaluation.
The National Medical Products Association (NMPA) maintains a database of devices which are exempt from submitting clinical data.
Click below for more insights
Additional insights related to clinical investigations of medical devices in China include:
- For clinical evaluations conducted with the clinical literature data and clinical data of the predicate device, the clinical evaluation materials shall include the comparison between the product under registration application and the predicate device, analysis and evaluation of clinical data of the predicate device, scientific evidence, and the evaluation conclusion, etc., submitted for the difference(s) between the product under registration application and the predicate device.
- For a clinical evaluation conducted through a clinical trial, the clinical evaluation materials shall include the clinical trial protocol, the ethics committee comments, the informed consent form, and the clinical trial report, etc.
- The clinical trial of medical devices shall be conducted according to the requirements of Good Clinical Practice (GCP) within a qualified clinical trial institution. Before the clinical trial, the sponsor shall file the clinical trial plan to the regulatory department of the province, autonomous region, and municipality directly under the central government where it is located.
- Where the clinical trial of a Class III medical device poses high risks, it shall be subject to approval by the NMPA at the national level.
- The review and approval of a clinical trial is a process whereby the NMPA conducts a comprehensive analysis to the degree of risks, clinical trial protocol, and a comparative analysis report on clinical benefit and risk that the device to be put on clinical trial may entail to determine whether to approve the clinical trial.
- The catalog of Class III medical devices subject to clinical trial approval shall be compiled, adjusted, and published by the NMPA. The clinical trials of Class III medical devices requiring review and approval shall be conducted within qualified tertiary A medical institutions.
Considerations related to the development and use of data generated outside of China include:
- For products already approved in other jurisdictions, documentation of the official certification in the other country/region must be submitted to in-country authorities by an appointed Chinese legal representative.
- For SaMD and digital tools that involve algorithms and AI/ML, an in-country study and clinical trial are required, even for SaMD products that were previously approved in other jurisdictions.
- For some Class III devices, foreign data may be permitted.
- Domestic and foreign medical devices are reviewed differently; the review processes for each are outlined below:
- Class II domestic medical devices shall be reviewed by the regulatory department of the provinces, autonomous regions, and municipalities directly under the central government, and the medical device registration certificate shall be issued after approval.
- Class III domestic medical devices shall be reviewed by the NMPA, and the medical device registration certificate shall be issued after approval.
- Imported Class II and Class III medical devices shall be reviewed by the NMPA, and the medical device registration certificate shall be issued after approval.
Engaging with national regulators
The federal regulatory agency is the National Medical Products Administration (NMPA). The NMPA is responsible for the regulation, registration, and safety supervision of medicines, medical devices, and cosmetics in China. The NMPA maintains the national registry of medical products and manages the approval of domestic Class III and imported Class II and Class III medical devices and the filing of imported Class I medical devices.
Within the NMPA, the Center for Medical Device Evaluation (CMDE) reviews and approves medical device applications.
Regulatory departments of provinces, autonomous regions, and municipalities under the central government play a large role in the regulatory review of medical devices. So it is important to understand which entities at the national or other levels will be engaged in the review of a medical device.
Regulations regarding medical device manufacture and distribution can be found on the NMPA website, including the regulation covering “Provisions for Medical Device Registration and Filing.”
In addition to standard medical device authorization pathways, China also provides the “Innovation Green Pathway” for the accelerated approval of innovative medical devices. To qualify for this pathway, a device must:
- Be a class II or III device with significant clinical value.
- Be a novel product and/or provide significant advancements.
- Possess a new patent or be under patent application review.
- Present a preliminary study with complete and traceable data.
The NMPA states that reviews of Class II devices for registration and change, or renewal of, registration should be completed within 60 working days. If supplementary information is requested, technical review will be completed within 60 working days of receipt of the supplemental information. For Class III devices, reviews for registration, change, or renewal of registration should be completed within 90 working days; similar to Class II, technical review will be completed within 60 working days of receipt of any supplemental information. Applicants should submit any requested supplemental information within 1 year of notification.
Medical device registrations are valid for 5 years; an application for renewal must be received 6 months prior to expiration of the current registration.
Further timeline information can be found in “Provisions for Medical Device Registration and Filing” and “Medical Device Supervision and Administration Regulations.”
Although the NMPA publishes contact information online, manufacturers typically work with local experts and agents to identify critical next steps.
Province/municipality/autonomous region-level medical product regulatory agencies are heavily engaged in the medical device authorization process. These entities are responsible for reviewing, approving, and registering domestic Class II devices, inspecting the quality management system for Class II and III devices, and supervising clinical trials and clinical trial institutions.











