

South Korea National Companion Guide
Pursue DHT market access in South Korea
Feedback
These pathways are constantly evolving. Work with us to keep them up to date by providing suggestions and feedback.
In South Korea, digital health technology (DHT) product regulatory authorization, value assessment, and pricing and reimbursement are conducted at the national level. However, developers often pursue patient access infrastructure via local routes.
What is a policy “full-stack”?
A policy “full-stack” provides a structured approach for integrating digital health technologies (DHT) into national healthcare systems.
Components of a comprehensive national policy for DHTs
Regulatory authorization
Product value assessment
Patient access infrastructure
Pricing & reimbursement
Read more about the core elements of a national policy “full-stack” approach.
This guide reflects current information as of 15 October 2024. National regulations and practices are subject to change and evolve, so developers are encouraged to regularly consult relevant agency websites for the latest information.
Explore DHT patient access and product scalability pathways
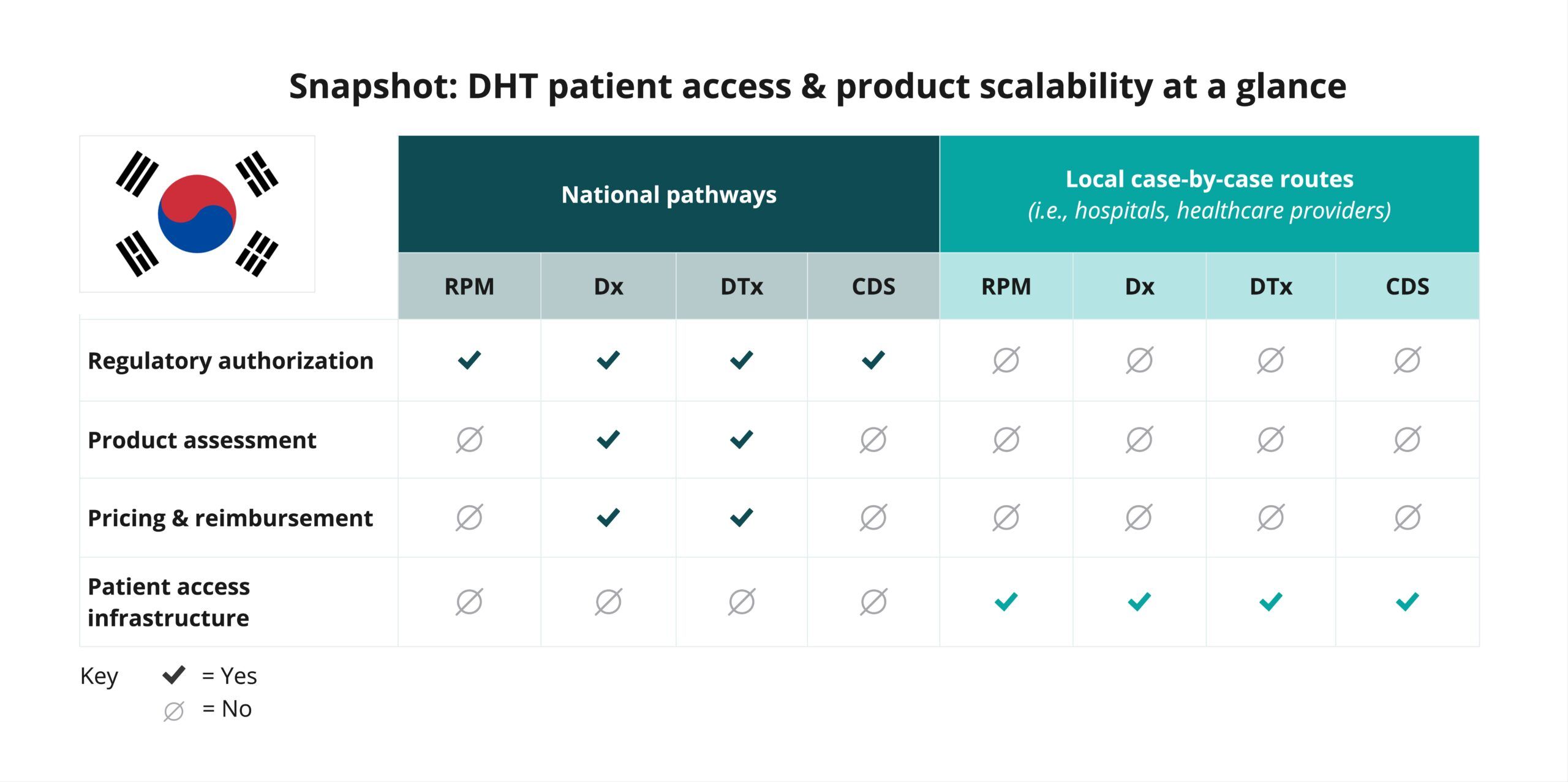
Begin your assessment of South Korea’s routes to DHT patient access by reviewing this snapshot, which offers insights into the potential patient access and product scalability pathways available for digital diagnostic (Dx) and digital therapeutics (DTx) products. Aside from a few exceptions, remote patient monitoring (RPM) and clinical decision support (CDS) products are not widely used in South Korea.
Navigate national pathways for DHT patient access
And then, use this flowchart to help you navigate South Korea’s national pathways for DHT market access, guiding you through key steps in regulatory review, product assessment, pricing, reimbursement, and patient access. Aligned with the four core elements of a national policy “full-stack,” it focuses on national pathways and does not provide detailed insights on local routes.
As you move through the flowchart, you’ll see where developers need to generate or present clinical evidence to demonstrate each product’s safety, efficacy, and impact. “Clinical evidence evaluation” refers to a review of published data, while “clinical investigation” highlights when you may need to conduct a study to verify your product’s safety and performance.
Use this flowchart to stay informed at each stage and understand what’s expected to bring DHTs to patients in South Korea.
This flowchart:
- Provides a sample of important steps along the pathway to patient access—not including post-market assessment pathways—and is subject to change. Other exclusions may exist.
- References DHT products that qualify as a medical device. DHT products that are not recognized as medical devices are not represented in this flowchart.
- Focuses primarily on national pathways and does not provide detailed insights on local case-by-case routes.
- Identifies certain opportunities to generate clinical evidence evaluations and investigations but does not incorporate health economic outcomes research (HEOR).
- Does not include specific timelines and is not intended to indicate how long or when steps are conducted.
National policy “full-stack” components
As indicated in the snapshot, DHT product regulatory authorization, value assessment, and pricing and reimbursement in South Korea are conducted at the national level.
Click below for more insights
- In South Korea, three regulatory agencies—Ministry of Food and Drug Safety (MFDS), Health Insurance Review and Assessment Service (HIRA), and National Evidence-based healthcare Collaborating Agency (NECA)—collaborate:
- MFDS oversees product approval and Korea Good Manufacturing Practice (KGMP) certification under the Medical Devices Act.
- HIRA assesses technologies based on the National Health Insurance Act.
- NECA evaluates the clinical and economic evidence of the product under the Medical Service Act.
- HIRA determines product reimbursability and establishes the reimbursement price for the medical device based on its assessment.
- In South Korea, remote patient monitoring (RPM) and clinical decision support (CDS) products are not typically used.
- Digital diagnostics (Dx) and digital therapeutics (DTx) products that qualify as a medical device can be classified into Class II, III, or IV of the four available product categories: Class I, II, III, or IV.
- Recent legislative and regulatory developments include:
- In 2020, MFDS released Guidance on the Review and Approval of Digital Therapeutics (DTx) related to the authorization of products that provide evidence-based therapeutic interventions to patients for preventing, managing, or treating medical disorders or diseases.
- In August 2023, the Ministry of Health and Welfare (MOHW) and HIRA introduced a temporary reimbursement framework for certain digital health technologies (DHTs) approved by MFDS and prescribed by healthcare providers. This framework allows patients access to select DTx while formal reimbursement pathways are being established.
- In December 2023, the National Assembly enacted the Digital Medical Products Act, with key provisions taking effect in January 2025.
- Formal pathways for the review and reimbursement of DHTs and digital medical devices (DMDs) at the national level continue to be developed.
Curated overview of clinical evidence requirements and practices
Clinical evidence requirements in South Korea are legislated by the Digital Medical Products Act (legislative language provided in Korean), which is due to be in effect as of January 2025.
Click below for more insights
- An entity that intends to conduct a clinical trial with a digital medical device shall prepare a clinical trial plan to be approved by the MFDS. The same applies to protocol changes.
- An entity that intends to conduct a clinical trial with a digital medical device shall conduct a clinical trial at a clinical testing center designated in accordance with Article 10(3) of the Medical Device Act.
- However, clinical trials falling under certain subparagraphs may be conducted at an institution other than the clinical trial site as prescribed by the Prime Ministerial Decree. In this case, the approval of MFDS must be obtained in advance, and the same applies to any changes to the approved plan. These situations include:
- A clinical trial where subjects can only be recruited from institutions that fall under any of the following sub-items:
- Infectious disease management institutions designated pursuant to Article 36(1) and (2) of the “Infectious Diseases Control and Prevention Act.”
- Medical institutions, isolation wards, nursing homes, or clinics that are designated, established, and operated as infectious disease management institutions pursuant to Article 37(1) of the “Infectious Diseases Control and Prevention Act.”
Engaging with national regulators
The Ministry of Food and Drug Safety (MFDS) regulates the safety of food, drugs, cosmetics, and medical devices to protect public health in South Korea. The Health Insurance Review and Assessment Service (HIRA) and the National Evidence-based healthcare Collaborating Agency (NECA) are the two primary entities DHT developers will engage with for product assessment, pricing, and reimbursement.
The health security system in Korea has two components: mandatory social National Health Insurance (NHI) and medical aid. NHI provides healthcare coverage to all citizens. The major sources of NHI funding include contributions from those who are insured and government subsidies. The medical aid program is a form of public assistance that uses government subsidies to provide low-income groups with healthcare services.
The Ministry of Health and Welfare (MoHW) oversees the NHI system and its two fundamental institutions: National Health Insurance Service (NHIS) and HIRA. NHIS serves as the insurer and HIRA conducts claims reviews, reimbursement determination for services and items (including medical technologies), and quality assessment of health care services.
In line with HIRA’s work, NECA is the national research agency that provides information about medical devices, medicines, and health technology through objective and reliable analysis. NECA contributes to the efficient use of national medical resources and the protection and enhancement of national health by proposing scientific evidence. NECA has two main bodies: the core research body, which focuses on health technology assessment (HTA) and collaboration research, and the Center for New Health Technology, which supports the Committee for New Health Technology Assessment.
A marketing authorization holder (MAH) must submit the medical device registration application(s) and hold the license thereafter. An overview of the medical device approval process can be found on the MFDS website. Nonetheless, the most up-to-date information can be acquired through your MAH, as they need to be in compliance with the local laws and regulations, or through a qualified medical device consultancy firm.
Product review timelines differ based on medical device risk classifications and pathways for a marketing authorization.
A sample conventional progression may include:
- Medical device approval by MFDS (80 days).
- Review of reimbursement eligibility by HIRA (30-60 days).
- New health technology assessment (nHTA) by NECA (140-250 days).
- Register for insurance benefit by the National Health Insurance Review & Assessment Service (100 days).
However, in practice, timelines may be significantly longer than what the statutory review periods state due to reviewers’ schedules.
NECA, aware of the medical device industry’s belief that the traditional review timeline is too long when conducted in sequence, implemented a simultaneous review of New Health Technology Assessment and Healthcare Coverage Determination to reduce the maximum duration from 490 days to 390 days. The goal of this process is to expedite the entry date of new health technology to the clinical environment, broaden the treatment choice of the patients, and contribute to developing and expanding the healthcare market.
General questions can be submitted to the FDA by email. The FDA aims to respond, or acknowledge receipt and provide a timeline for response, within 5 days of receipt.
Does the agency encourage a pre-submission OR scientific advice process or discussion? If yes, how does a company initiate this process?
Yes, the FDA encourages pre-submission discussion. Companies may contact the FDA through the Q-Submission Program, which is a pathway for receiving formal feedback from the FDA prior to product submission, either written or in a meeting. Please refer to the guidance document outlining the Q-Submission process.
The FDA’s main support structure for regulatory applications is through the Q-submission process, outlined above. The agency will engage with companies during the submission process as outlined in the guidance documents for each pathway.
The majority of applications can be submitted online through the FDA’s Center for Devices and Radiological Health (CDRH) portal. As of October 1, 2023, all 510(k) submissions must be made through using the FDA’s electronic Submission Template and Resource (eSTAR). At this time, De Novo requests may be made electronically through eSTAR or by mail through eCopy (instructions), while PMA submissions must be made through eCopy. Q-submission and Breakthrough Device Designation requests are also made through the CDRH portal.
Glossary of terms
Clinical performance test
Test or study that analyzes a specimen to predict clinical, physiological, or pathological conditions, or confirm the results to prove the performance of a digital medical device.
Source: Digital Medical Products Act
Digital combination/convergence drug
Pharmaceutical product combined with a “digital medical device” or a “digital medical and health support device.” However, this classification excludes cases where the main function of the product is as a digital medical device.
Source: Digital Medical Products Act
Digital medical and health support device
Device, machine, device software, or similar product that is not a digital medical device, but is used to monitor, measure, collect, and analyze vital signs for the purpose of supporting medical care or maintaining or improving health, or to provide health information such as diet and exercise by recording and analyzing lifestyle habits, and is designated by the Minister of Food and Drug Safety.
Source: Digital Medical Products Act
Digital medical device
Medical device or a combination of a digital medical and health support device which incorporates advanced technologies such as intelligent information technology, robotics technology, and/or information and communication technology which falls under any of the following categories:
- Used for the purpose of diagnosing, treating, or monitoring the prognosis of a disease.
- Used for the purpose of predicting the response to treatment and outcome of a disease.
- Used for the purpose of monitoring the effectiveness of treatment or side effects of a disease.
- Other products, designated by the Commissioner of Food and Drug Safety, as used for the purpose of assisting rehabilitation.
Source: Digital Medical Products Act
Digital medical products
Digital medical devices, digital combination/convergence drugs, and digital medical and health support devices.
Source: Digital Medical Products Act
Medical device
Instrument, machine, apparatus, material, software, or any other similar product used, alone or in combination, for human beings or animals for one of the following purposes:
- Diagnosing, curing, alleviating, treating, or preventing a disease.
- Diagnosing, curing, alleviating, or correcting an injury or impairment.
- Testing, replacing, or transforming a structure or function.
- Control of conception.
Excludes drugs, quasi-drugs (regulated under the Pharmaceutical Affairs Act), prosthetic limbs, and assistive devices for people with disabilities (regulated under Article 65 of the Act on Welfare of People with Disabilities).
Source: Korean Medical Devices Act
Modified device
Medical device that is equivalent in “Intended Use,” “Mechanism of Action,” and “Raw Materials,” with previously cleared/approved/certified/notified medical devices, but not equivalent in “Performance,” “Test Specification,” or “Instructions for Use.”
Source: MFDS
New (novel) device
Medical device that is not equivalent in “Intended Use,” “Mechanism of Action,” or “Raw Materials” with previously cleared/approved/certified/notified medical devices.
Source: MFDS
Substantially Equivalent (SE)
Medical device that is equivalent in “Intended Use,” “Mechanism of Action,” “Raw Materials,” “Performance,” “Test Specification,” and “Instructions for Use” with previously cleared/approved/certified/notified medical devices.
Source: MFDS
Wellness device
Any instrument, machine, material, software, or application which is intended to be used for human beings by itself or in combination with others for the purpose of:
- Maintaining or improving a general healthy condition or activity.
- Inducing a healthy lifestyle or habit.
- Supporting self-management for chronic diseases (e.g., cardiac disorder, hypertension, hypotension, diabetes, etc.).
Acknowledgements
- D-Health Consulting
- Lunit Inc.
- MDREX, Medical Device and Digital Health Consulting Group
- WELT Corp












