Laying the Foundation: Defining Digital Medicine
The Digital Medicine Society (DiMe) was founded on a very deliberate definition of digital medicine, developed by a diverse and interdisciplinary group of experts (thank you to our esteemed Strategic Advisory Board and Scientific Leadership Board!).
This definition of digital medicine is the cornerstone of DiMe and our activities to advance the field. I have read this definition a thousand times, and every time I am excited by the possibility of digital medicine improving health, health outcomes, health economics, and increasing access to care. I’m also reminded of the fragile nature of that possibility; how we must thoughtfully develop the field of digital medicine to ensure that the products we place our trust in are, indeed, trustworthy.
Creating this definition establishes a common language and framework upon which to build the field of digital medicine. It’s the first of many steps that the DiMe community is taking to advance digital medicine to optimize human health. Join us.
What is Digital Medicine?
Digital medicine describes a field that uses technologies as tools for measurement and intervention in the service of human health. Digital medicine products are driven by high-quality hardware and software that support the practice of medicine broadly, including treatment, recovery, disease prevention, and health promotion for individuals and across populations.
Digital medicine products can be used independently or in concert with pharmaceuticals, biologics, devices, or other products to optimize patient care and health outcomes. Digital medicine empowers patients and healthcare providers with intelligent and accessible tools to address a wide range of conditions through high-quality, safe, and effective measurements and data-driven interventions.
As a discipline, digital medicine encapsulates both broad professional expertise and responsibilities concerning the use of these digital tools. Digital medicine focuses on evidence-generation to support the use of these technologies.
Why Now?
The rise of software-driven connected technologies has created a fundamental shift in health data and information flows, and thus in the practice of medicine.
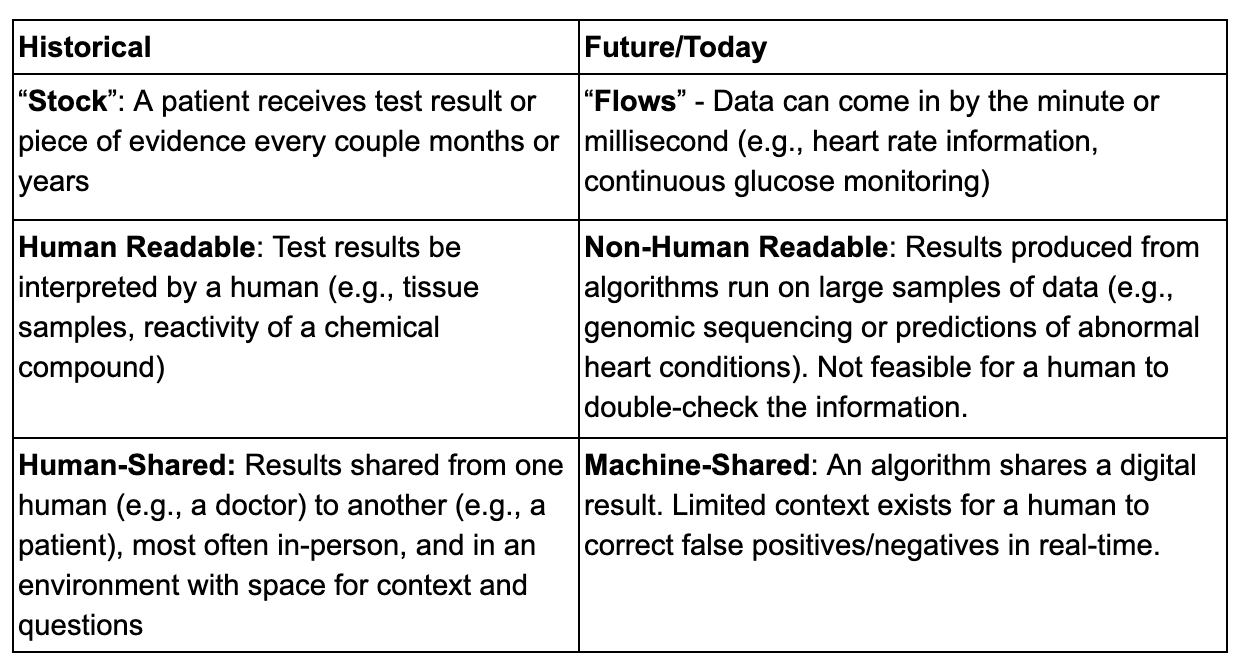
In the digital era of medicine, a number of processes, best practices, and ethical norms have started to shift as we learn how to develop trustworthy technologies. Sample paradigm shifts include the need to develop new methods to determine verification and validation, clinical usability and human experience, cybersecurity risks, and data rights and governance from ‘digital specimen’ collection.
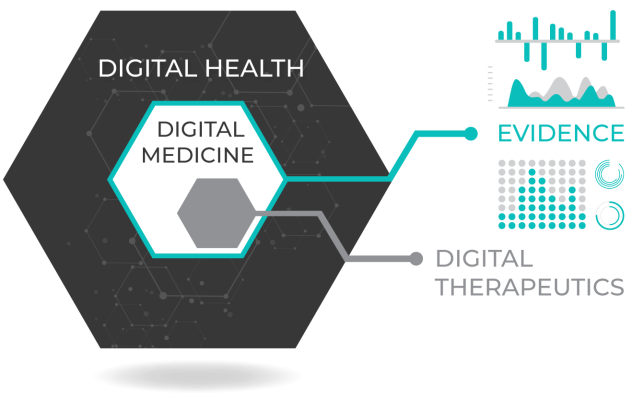
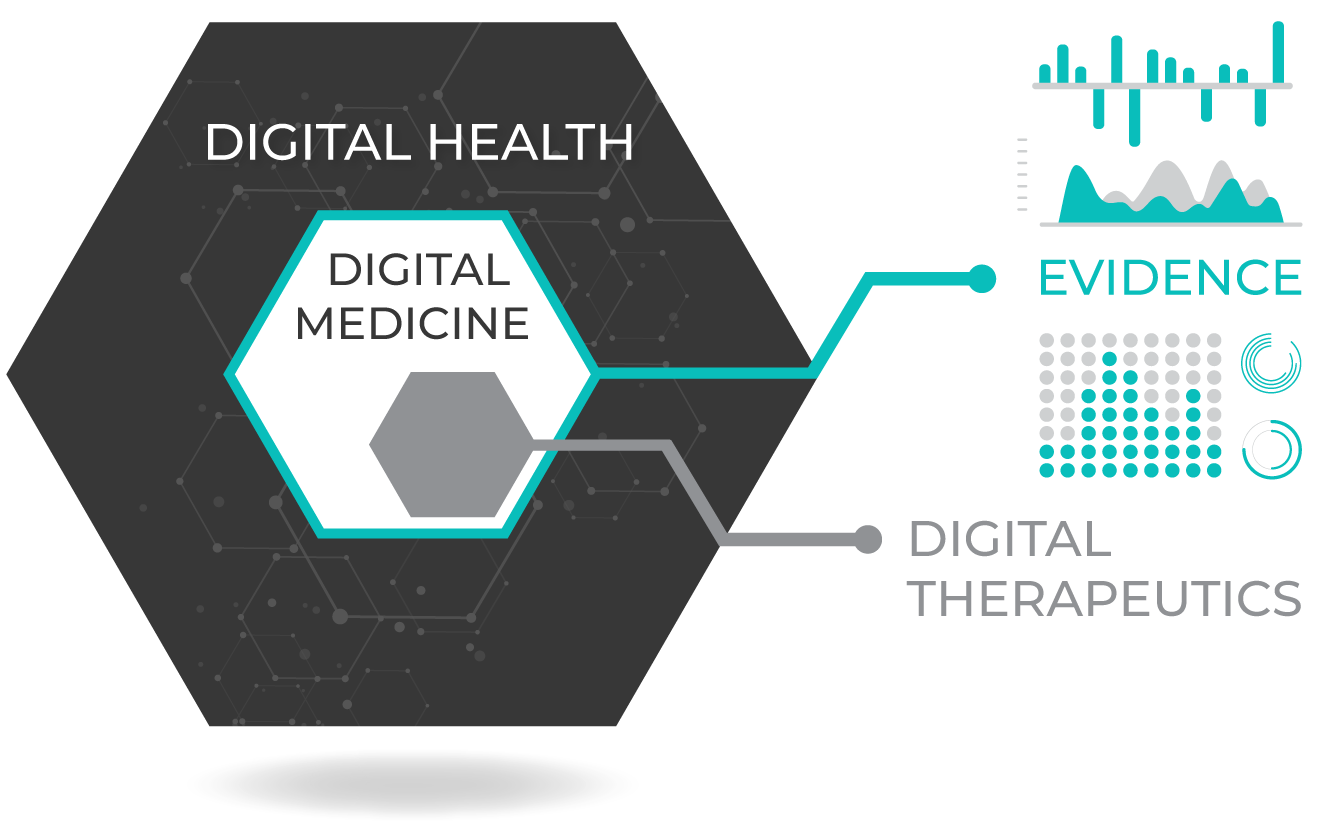
As digital medicine evolves, we must ensure that these technologies are worthy of the trust we place in them. Unlike “digital health” or “digital wellness” products, digital medicine products are characterized by a body of evidence to support their quality and effectiveness.
Who Practices Digital Medicine?
The same individuals that practice traditional medicine: from pharmacists to physicians and nurses to health navigators. We also include medical researchers, from physician investigators to experts studying behavioral and population health.
Critically, we recognize the expanded role of the patient in the practice of digital medicine. With digital tools providing opportunities to decentralize care outside of the hospital, clinic and lab, patients will have a much more active role in digital medicine. Using digital tools in research will also expand the role of the citizen scientist.
Who Are the Key Stakeholders in Digital Medicine?
- Patients are the stakeholders at the center of digital medicine, and we also recognize the critically important role of caregivers.
- Clinical care providers remain at the center of medicine and are ultimately the experts who drive decision making at the point of care.
- Physician-scientists are central to the discovery and development of novel measurements and treatments and are responsible for the ethical and safe conduct of research.
- Healthcare researchers design and execute studies that either provide the substrate for future digital development or examine digital tools themselves.
- Regulators and payers will be as influential in digital medicine as they are in the development and practice of, as well as access to, traditional medicine.
- Developers of traditional (non-software) medical products — biopharma and medical device companies — are stakeholders in this field as the line between ‘traditional’ and ‘digital’ medicine continues to blur.
- Experts in the emerging field of digital therapeutics will take on a pivotal role in developing new interventions to improve clinical and health economic outcomes.
- Data scientists will play an increasing role as the density of data available from digital data tools revolutionized our industry. Similarly, cyber-security experts and ethicists will have critical roles in this new, digital era in healthcare.
- Engineers — both hardware and software — will be central to the advancement of digital medicine, providing the tools this industry will be built on. They will need to work closely with other stakeholders to understand the nuances of a highly regulated industry built on individuals’ health data.
- Funders — both government and non-governmental funding organizations that support health research will have significant impact on the direction and pace of the evolution of digital medicine.
How Will Digital Medicine Evolve over Time?
When we founded this society, we were inspired by the FDA-NIH Framework, that stated: “Effective, unambiguous communication is essential for efficient translation of promising scientific discoveries into approved medical products. Unclear definitions and inconsistent use of key terms can hinder the evaluation and interpretation of scientific evidence and may pose significant obstacles to medical product development programs.”
This manifesto is our working draft to drive the field forward. Like any piece of software, it is a living product that requires maintenance and updates to maintain its relevance. The role of the society is to maintain a focus on the areas of the field that need the most attention, clarification, and guidance, and we will continue to refine frameworks and definitions to support those developing and adopting digital medicine technologies.
What are Examples of Digital Medicine Products?
Digital medicine products are evidence-based tools that support the ‘practice of medicine.’
Measurement products include digital biomarkers (e.g., using a vocal biomarker to track change in tremor for a Parkinson’s patient), electronic clinical outcome assessments (e.g., a electronic patient-reported outcome survey), and tools that measure adherence and safety (e.g., a wearable sensor that tracks falls).
Intervention products include digital therapeutics and connected implantables (e.g., an insulin pump). Digital therapeutics deliver evidence-based therapeutic interventions to patients that are driven by high quality software programs to prevent, manage, or treat a medical disorder or disease. They are used independently or in concert with medications, devices, or other therapies to optimize patient care and health outcomes.
Combination products both measure and intervene. For example, continuous glucose monitors (CGMs) for diabetics share patient data automatically with their doctor’s office using a companion app. The level of human involvement may vary in the cycle between measurement and intervention — say, when a doctor diagnoses an abnormal heart condition from an EKG reading from a smartphone. Over time, this cycle may become more closed-loop, with less need for human intervention in response to routine changes. More recently, the development of the “artificial pancreas” has combined the CGM with an insulin pump and a computer-controlled algorithm that allows the system to automatically adjust the delivery of insulin to reduce high blood glucose levels (hyperglycemia) and minimize the incidence of low blood glucose (hypoglycemia).



